Global warming has been one of the hot topics in recent years and has received widespread attention. Temperature, as an important environmental factor, participates in regulating many aspects of plant growth and development and has a very important impact on agricultural production. Therefore, the study of temperature perception and signal transduction mechanism will help us provide a theoretical basis for subsequent molecular breeding. The mechanism of plant thermal perception has always been a frontier field of plant research, especially the search for plant thermoreceptors is the most important topic in this field. In recent years, breakthrough progress has been made in the research mechanism of plant thermal perception, mainly represented by the following two Nature articles.
On May 15, 2024, Nature published a research paper titled "The temperature sensor TWA1 is required for thermotolerance in Arabidopsis" by the teams of Erwin Grill and Alexander Christmann from the Technical University of Munich. The study revealed that TWA1 is a temperature-sensing transcriptional co-regulator, which is required for basal and acquired heat resistance in Arabidopsis thaliana.
The study showed that TWA1 is a disordered protein, and its key temperature sensing function is exerted through the N-terminal highly variable region. Under high temperature, TWA1 in plants changes conformation and then interacts with JASMONATE-ASSOCIATED MYC-LIKE (JAM) transcription factors and TOPLESS (TPL) and TOPLESS-RELATED (TPR) to assemble into an inhibitory complex.
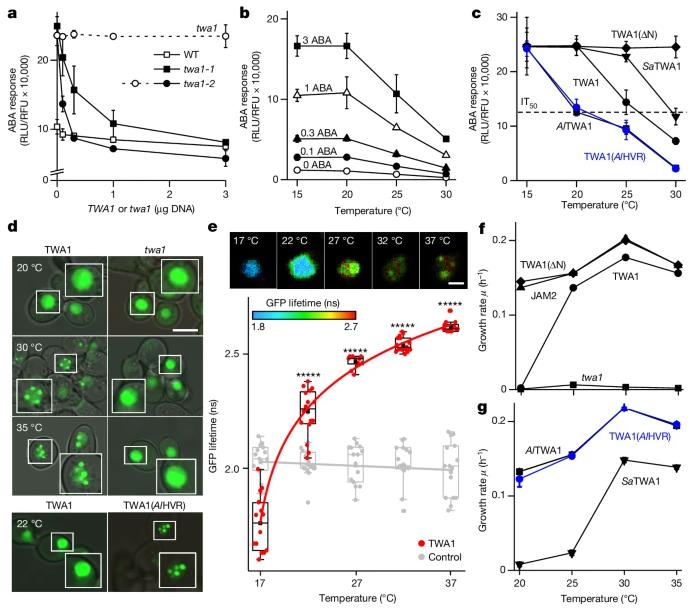
Figure 1. The thermosensor function of TWA1. (Jung, et al., 2024)
In July 2020, Nature published a research paper titled "A prion-like domain in ELF3 functions as a thermosensor in Arabidopsis" by the team of Philip A. Wigge from the University of Cambridge. The study revealed that the ELF3 protein in Arabidopsis contains a predicted prion domain (PrD), which can sense the external temperature by phase transition at high temperatures to quickly switch EARLY FLOWERING 3 (ELF3) between active and inactive states. Therefore, the article revealed that the PrD domain in the ELF3 protein is a thermosensor in plants.
In Arabidopsis, the core component of the biological clock, the Evening Complex (EC), inhibits the transcription of PIF4/5 at night, causing the growth rate of the plant hypocotyl to peak at dawn. It is composed of three proteins, including scaffold proteins and temperature-responsive ELF3, small α-helical protein ELF4, and DNA-binding protein LUX ARRYTHMO (LUX). Among them, ELF3 contains polyglutamine (polyQ) repeat sequences. In addition, previous studies have found that the length of polyQ repeats contained in ELF3 in various Arabidopsis ecotypes in natural populations varies from 7 to 29, and is associated with phenotypic variation.
The authors first studied whether the length of polyQ repeats affects ELF3 activity? The results showed that the longer the length of ELF3 transgenes complementing different polyQ lengths in the elf3-1 mutant, the better the thermosensitivity, indicating that the PrD of ELF3 plays a role in the temperature perception of Arabidopsis. In addition, the study found that compared with Arabidopsis, StELF3 of potato grown in a temperate climate has a smaller predicted PrD, while BdELF3 of Brachypodium distachyon is not predicted to have a PrD region, and both proteins cannot be thermoresponsive in Arabidopsis at 27 °C. In addition, the chimeric ELF3-BdPrD showed inhibition of temperature-responsive flowering, confirming that the PrD from Arabidopsis is thermoresponsive. Since ELF3 is a temperature-dependent transcriptional repressor, the study further confirmed that as the temperature rises, the occupancy of ELF3 on the target gene also changes, and this process depends on PrD, indicating that PrD directly regulates the thermal responsiveness of ELF3 binding on the target gene.
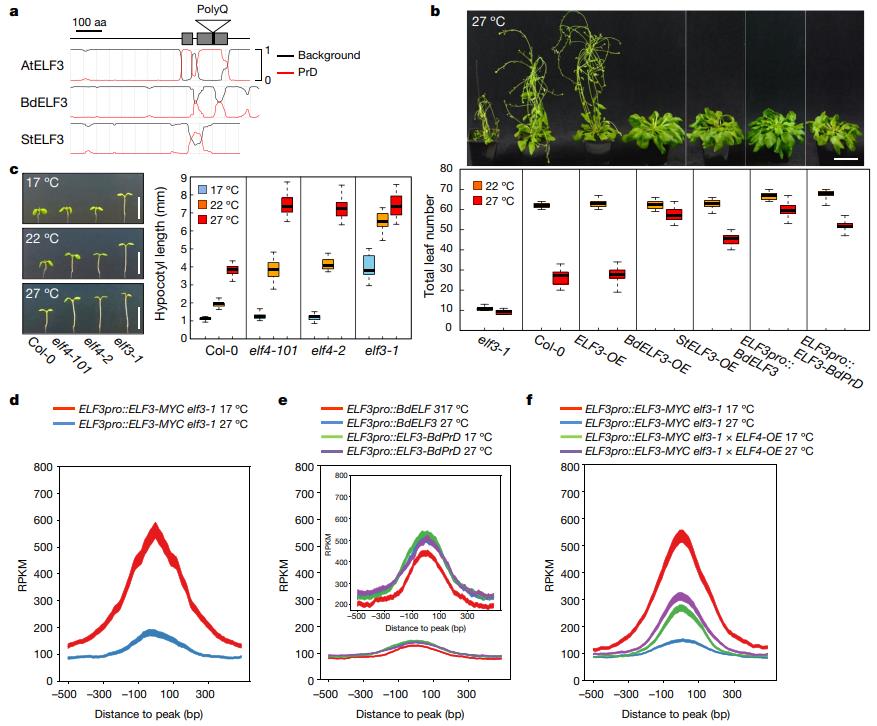
Figure 2. Poly Q is essential for thermal response and is regulated by ELF4. (Bohn, et al., 2020)
The study then explored whether temperature directly regulates ELF3 activity? Therefore, the study constructed ELF3-GFP fusion protein transgenic plants. The study found that ELF3-GFP had a diffuse signal at 17°C, while multiple bright spots formed at 35°C, and the increase in polyQ length also led to a greater tendency to form spots. Further studies purified ELF3 PrD fused to GFP (PrD–GFP). The study found that ELF3 PrD was more soluble at low temperatures, while lowering the salt concentration and pH value induced a phase transition of PrD-GFP, forming micron-sized spherical droplets that separated, with a midpoint temperature of 28.7±1.8°C. The above results show that ELF3 can adopt two conformations, an active soluble form, and a high-order multimer form in which bright spots can be seen at warmer temperatures, both of which are reversible.
The two studies have similar mechanisms, both of which involve receptor proteins rapidly switching between active and inactive states to sense temperature changes, a previously unknown mechanism of heat sensing.