Helianthus annuus L., also known as sunflowers, are divided into two categories: edible and ornamental varieties. Sunflower is the world's third most important oil-producing crop. Its seeds contain high oil content and are delicious. With the development of agricultural economy, sunflower, as a common product in the agricultural market that mainly is eaten or squeezed to oil, has a strong market demand, which provides a source of power for the development of the sunflower industry. But with the improvement of our living standards, there are higher requirements for the quality of sunflower products. Therefore, genetically modified sunflower came into being. In recent years, researches on Helianthus annuus L. gene modification have continued to emerge, including speeding up the seed germination process, changing the color of leaves, and improving disease resistance, insect resistance, herbicide resistance, salt tolerance, cold tolerance, storage tolerance, seed flavor, yield, etc.

Lifeasible provides one-stop services, covering all steps from experimental design, vector construction, plasmid transformation, positive transplant screening to characterization of transgenic Helianthus annuus L. We offer Helianthus annuus L. transformation using various genetic engineering technologies as follows:
Through the quantitative overexpression of genes related to Vitamin E, phytosterols, phospholipids and other chemical components in Helianthus annuus L., the production of specific compounds can be increased. We could help you overexpress many enzymes expression related genes like drought resistance, salt tolerance genes P5CS, CL5734, HERC2, antioxidant protein expression gene SsPrxQ, disease-related protein gene HaPR1 that helps to increase the activity of SOD, POD and CAT, and drought stress gene AFLP during seed germination and many other genes related to important traits of Helianthus annuus L.
RNAi could induce specific mRNA degradation using homologous double-stranded RNA (dsRNA), then silence specific gene. Through RNAi technology, the silencing of multiple genes in Helianthus annuus L. can be mediated.
The VIGS technology is a transient transformation method which uses viruses carrying target gene fragments to silence endogenous genes of target plant. The silencing of some genes may lead to phenotype change, providing information for study of gene function. Due to the short period, VIGS can help you get valuable information for gene functional analysis fastly. With wealth of experience in VIGS, the scientists in Lifeasible provides you customized protocol for VIGS in Helianthus annuus L. We could achieve the transformation for Helianthus annuus L. with different genetic backgrounds.
The discovery of the CRISPR/Cas9 system provides us with a very powerful and convenient gene editing tool. By using CRISPR technology, we can achieve knockout of Helianthus annuus L. genes in different ways, including frameshift mutations, multiple deletion of fragments, knockout of non-coding genes, knockout of multiple copies of genes, etc.
Using the powerful scalability of the CRISPR system, we have developed many methods that can improve gene knock-in efficiency and achieve precise editing of the Helianthus annuus L. genome. Though NHEJ and HDR may occur at the same time due to DNA breaks, however, most of CRISPR gene knock-in is done through HDR. Therefore, we have developed different methods to increase the probability of HDR, thereby improving the efficiency of gene knock-in. Besides, prime editing, a technology could realize any type of base substitution is available at Lifeasible.
Through CBE and ABE, we could help you achieve the conversion from C to T or A to G in Helianthus annuus L. without DNA double strand break. During single base editing, the C base deaminase or A base deaminase is located at a specific position in the genome, and it catalyzes the deamination reaction of C or A at a specific position and turns it into U or I. Then it is treated as T or G in the process of DNA replication, realizing the conversion from C to T or A to G.
With the development of CRISPR/Cas9 technology, modifications of the CRISPR system have endow them with more utility function, one of them is CRISPRi technology. CRISPRi technology can inhibit the transcription of designated targets, whether it is coding or non-coding DNA fragments. CRISPRi uses catalytically inactivated Cas9 (dCas9). When dCas9 binds to the genome, it will block the binding of the transcription machinery and realizing Inhibition of gene transcription through steric hindrance. For the inhibition of Helianthus annuus L. genes, we can provide a variety of solutions, including dCas9 binding to targeted DNA and realizing Inhibition of gene transcription through steric hindrance. In addition, gene knockdown of Helianthus annuus L. can also be achieved by recruiting a fusion inhibiting protein to the start site of gene transcription.
CRISPRa technology is very suitable for enhancing the expression of specific genes or analyzing the function of specific genes. For Helianthus annuus L. genes, we provide VPR technology, SAM technology and Suntag technology to make CRISPR system carry more activation element and achieve stronger activation effect after synergistic amplification.
Genetic screening system based on CRISPR technology can complete mutation library construction work at a very low-cost. The gene mutation library construction technology we provide for Helianthus annuus L. including gene knockdown library construction, gene knockout library construction, and gene activation library construction. Moreover, single-cell sequencing is available for mutation screening.
DNA-free gene editing has received extensive attention in recent years. It can effectively prevent genetic pollution and gene transfer, and has high biological safety. We provide DNA free Helianthus annuus L. genome editing services, including heterochromosomal gene editing, transient expression of CRISPR/Cas9 plasmid DNA, in vitro transcription of CRISPR/Cas9, and pre-assembled ribonucleic acid composed of purified Cas9 protein and sgRNAs complex. These technologies can avoid the integration of foreign DNA and genome, and reduce off-target effects. In addition, compared with traditional techniques, these techniques can avoid the use of hybridization or backcrossing to isolate CRISPR/Cas9 chimeras, so they are cheaper and have shorter experimental cycles.
Genetic Transformation Process for Helianthus annuus L.
So far, the most advanced and widely used method for the development of genetically modified Helianthus annuus L. is Agrobacterium-mediated. Briefly, seedlings with one cotyledon freshly removed were submerged in the Agrobacterium suspension for 5 minutes, and after foreign target gene is transferred and integrated into plant cells, transformed plants are regenerated through tissue culture.
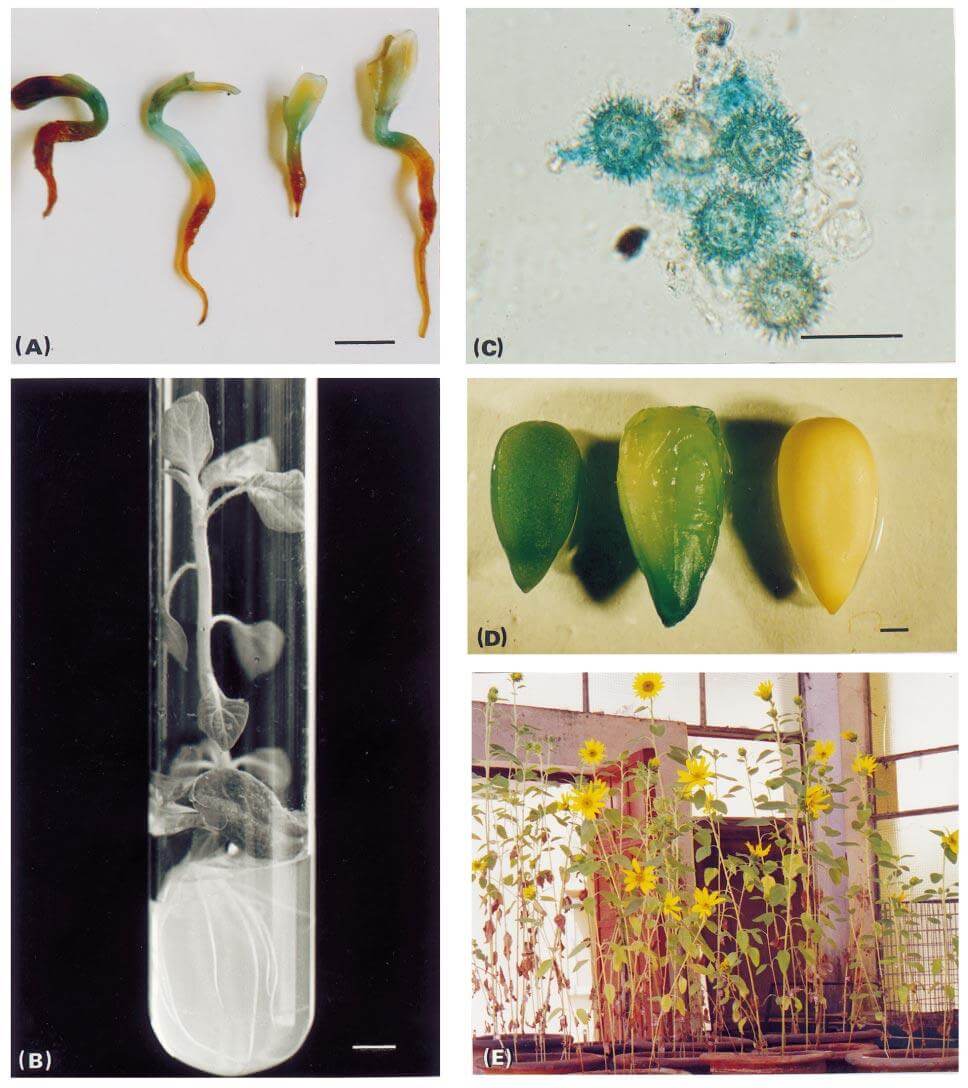 Figure 1. Stages in the production of transgenic plants of Helianthus annuus L., (A) Seedlings with one cotyledon freshly removed infected with Agrobacterium LBA 4404, (B) Kanamycin-selected plant 4 weeks after infection. (C) GUS expression in the pollen of primary transformants. (D) GUS expression in the seeds of transformed plants and lack of expression in the seed of uninfected plant (negative control). (E) T1 plants established in the glasshouse. (Sankara RK, et al. 2014)
Figure 1. Stages in the production of transgenic plants of Helianthus annuus L., (A) Seedlings with one cotyledon freshly removed infected with Agrobacterium LBA 4404, (B) Kanamycin-selected plant 4 weeks after infection. (C) GUS expression in the pollen of primary transformants. (D) GUS expression in the seeds of transformed plants and lack of expression in the seed of uninfected plant (negative control). (E) T1 plants established in the glasshouse. (Sankara RK, et al. 2014)
Lifeasible offers our customers with professional one-stop services, covering experimental design, vector construction, plasmid transformation, positive transplant screening and testing. Adapting to diverse purposes of different customers, multiple Agrobacterium strains (GV2260, C58, J2, K599, BGOH, JTND, LBA4404, EHA101, EHA105, etc.), as well as commercial and customized binary vectors with variant selectable markers (Kanamycin, Hygromycin, Phosphinothricin, G418, etc.) are readily available for your use. Experts at Lifeasible obtain comprehensive knowledge and years of experience to solve technical problems and challenges in Helianthus annuus L. transformation. We can draw customized solution to help you research on a variety of Helianthus annuus L. genes (Including drought resistance, salt tolerance genes P5CS, CL5734, HERC2, antioxidant protein expression gene SsPrxQ, disease-related protein gene HaPR1 that helps to increase the activity of SOD, POD and CAT, and drought stress gene AFLP during seed germination, etc.), Our services guarantee the success of your project. For more information or any inquiry requirements, please contact Lifeasible.
Reference