Endoplasmic Reticulum Quality Control for ER Stress in Plants
Endoplasmic reticulum quality control (ERQC) is mainly responsible for monitoring and controlling the folding state of proteins in the endoplasmic reticulum (ER). Protein folding is closely related to the N-chain protein glycosylation modification process of the primary peptide chain. The sugar structure added to the primary peptide chain of the protein is called G-oligosaccharide (Glc3Man9GICNAc2). Glycoproteins undergo a folding process, including CNX / CRT cycling, UGGT modification, and finally, misfolded proteins are removed.
Lifeasible provides services to customers worldwide in the field of endoplasmic reticulum quality control in plants. Our platform is equipped with advanced facilities and professional experts to support related research. Here we provide various services according to customer needs.
CNX / CRT Cycling in Endoplasmic Reticulum Quality Control
- The G-oligosaccharide structure on the peptide chain is recognized by the molecular chaperone CNX on the ER membrane and its cognate protein CRT located in the ER lumen. CNX / CRT can recruit a large number of molecular chaperones that help fold protein as well as peptide chains correctly.
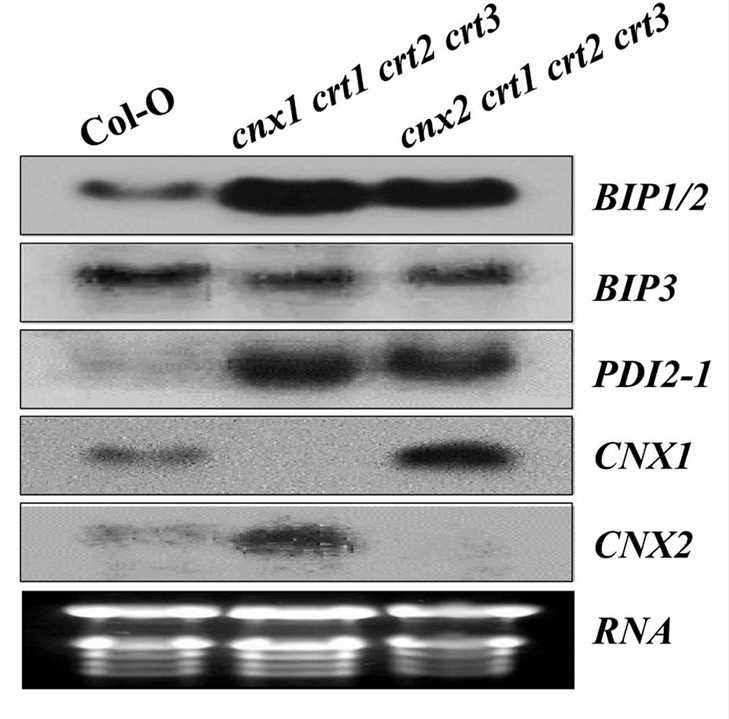 Fig.1. Expression pattern of ER stress-responsive genes in wild-type and two quadruple mutant plants. (Vu KV, et al., 2017)
Fig.1. Expression pattern of ER stress-responsive genes in wild-type and two quadruple mutant plants. (Vu KV, et al., 2017)
- Lifeasible helps our customers study this process through the following methods in plants, containing development and generation of plant mutant, RNA extraction and expression analysis, RNA blot analysis, treatment with tunicamycin (TM) and dithiothreitol (DDT), and others.
- We provide the solutions for experiments in which wild-type plants and mutants are grown on media containing a range of TM and DTT concentrations that cause misfolded proteins to accumulate in the ER. The expression levels of ER stress response genes such as BiP1 / 2, BiP3, and PDI2-1 are observed by RNA blot analysis.
UGGT Modification in Endoplasmic Reticulum Quality Control
- UDP glucose glycoprotein glucosyltransferase (UGGT) is a protein localized in the endoplasmic reticulum lumen. Its N-terminal structure can bind to the incorrectly folded protein structure, and its C-terminal is the glycosyltransferase catalytic domain. As a protein folding state detector, UGGT can ensure the maximum possible protein folding.
- Lifeasible helps our customers study this process through the following methods in plants, containing the development of UGGT mutant, preparation of plant ER-enriched vesicles, UGGT activity, quantitative PCR, and others.
- To assess the sensitivity of the UGGT mutants to the ER stress, we perform the sensitivity of the two mutants to these compounds in the presence of ER stress inducers. In addition, the transcription levels of BIP1 / 2, BIP3, and PDIL2-1, as well as AtUTr1, the genes encoding ER-localized UDP-glucose transporter, are also quantitatively analyzed in real-time.
Lifeasible provides cost-effective, high quality, and comprehensive services to our clients worldwide. We provide our clients with direct access to our experts as well as prompt response to their inquiries. If you are interested in our services or have any questions, please feel free to contact us or make an online inquiry.
Reference
- Vu KV, et al. (2017). "Systematic deletion of the ER lectin chaperone genes reveals their roles in vegetative growth and male gametophyte development in Arabidopsis." Plant J. 89 (5), 972-983.
For research or industrial raw materials, not for personal medical use!
 Fig.1. Expression pattern of ER stress-responsive genes in wild-type and two quadruple mutant plants. (Vu KV, et al., 2017)
Fig.1. Expression pattern of ER stress-responsive genes in wild-type and two quadruple mutant plants. (Vu KV, et al., 2017)