Functional Analysis of the Protein Body
Seed storage proteins are stored in two different cellular structures; one is the protein body (PB), and the other is the protein storage vacuole (PSV). The protein body is highly specialized protein storage organelles in grain seeds. PB formation in seeds begins in the endoplasmic reticulum (ER). Depending on the plant species, PBs remain in or come out of the ER, bypassing the Golgi and eventually entering the protein storage vacuole.
Lifeasible is committed to helping our customers achieve effective and successful research. We provide convenient and guaranteed functional analysis of the protein body, including storing prolamins via protein-protein interactions, mRNA sequences, and autophagy. In addition, we deliver reliable results and reports on time to our customers worldwide.
Protein Body Stores Prolamins via Protein-Protein Interactions
- All prolamins are soluble in aqueous ethanol solutions, which reflects their general hydrophobicity. However, prolamins from the Triticeae (wheat, barley, and rye) and the Panicoideae (maize, sorghum, and millet) contain sulfur-poor and sulfur-rich types and possess a high percentage of proline and glutamine, hence the name prolamins.
- It has been shown that PBs are formed through specific interactions between sulfur-rich and thiol-poor prolamins.
- Lifeasible provides functional analysis, including recombinant DNA techniques, plant transformation and regeneration, prolamins extraction and immunoblot analysis, electron microscopy, and double-labeling with the antibodies of prolamins, to illustrate that co-expression of prolamins genes results in the formation of endoplasmic reticulum derived protein bodies.
Protein Body Stores Prolamins via mRNA Sequences
- In addition, translation products of prolamin mRNA are transported to the lumen of the ER, where they accumulate to produce protein aggregates, then they could form PB directly. The process has been shown to be mediated by cytoskeleton and proteins that interact with the 3' noncoding sequence of the mRNA.
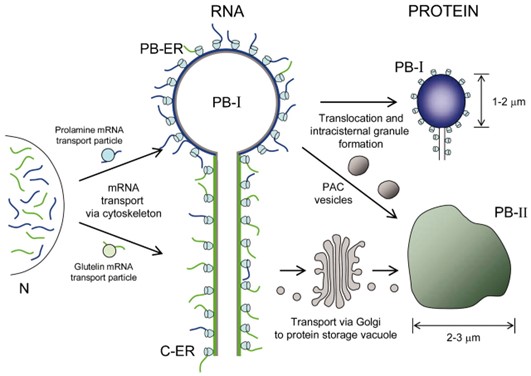 Fig.1. Schematic representation of RNA-dependent seed storage protein targeting in developing rice endosperm. (Crofts AJ. et al., 2004)
Fig.1. Schematic representation of RNA-dependent seed storage protein targeting in developing rice endosperm. (Crofts AJ. et al., 2004)
- We provide functional analysis, including subcellular fractionation, quantitation of glutelin and prolamin mRNAs, preparation of RNA probes for in vitro or in situ hybridization, in vitro hybridization of protein body fraction and in situ hybridization, to indicate that prolamin mRNA can also direct its synthesis.
Protein Body Stores Prolamins via Autophagy
- In some cereals, autophagy is also used to accumulate storage proteins, bypassing the conserved mechanism of Gorki-mediated targeting and transport to the vacuole.
- We mainly provide transgenic plants, electron microscopy, and immunocytochemistry services to meet the needs of our customers in this research.
Lifeasible has extensive experience and expertise in plant science. We are committed to providing you with timely and high-quality deliverables. At the same time, we guarantee the cost-effectiveness, completeness, and simplicity of the report. If you are interested in our services or have any questions, please feel free to contact us or make an online inquiry.
Reference
- Crofts AJ., et al. (2004). "Targeting of proteins to endoplasmic reticulum-derived compartments in plants." Plant Physiol. 136 (3), 3414-0.
For research or industrial raw materials, not for personal medical use!
 Fig.1. Schematic representation of RNA-dependent seed storage protein targeting in developing rice endosperm. (Crofts AJ. et al., 2004)
Fig.1. Schematic representation of RNA-dependent seed storage protein targeting in developing rice endosperm. (Crofts AJ. et al., 2004)