Bioequivalence Analysis of Herbal Medicines
Inquiry
The quality standards for herbal preparations are defined by the various tests, indicators, limits, and ranges of the preparations to ensure their quality. However, in the process of developing a drug, the question often arises as to whether the modified product has the same efficacy and safety as the product before the change. For this reason, it is necessary to carry out a quality assessment using a relevant method, i.e., a bioequivalence study. Bioequivalence is the main kinetic parameter that reflects the rate and extent of absorption of different formulations of the same drug given at the same dose under the same experimental conditions. Other preparations of the same herbal combination can only be used interchangeably if they are bioequivalent and have the same clinical efficacy and safety. Lifeasible's bioequivalence analyses play an essential role in the development and evaluation of medicines, helping you to determine whether your product can replace a marketed medicine.

- Pharmacological equivalence analysis
- Pharmacological pharmacodynamic equivalence analysis
- Clinical pharmacodynamic equivalence analysis
Parallel designs
In a parallel design, there is no need for crossover dosing, and inter-individual variation has a more significant impact on the trial. We would recommend a parallel design for drugs with long elimination half-lives or high variability.
Crossover designs
As most drugs have different clearance rates between individuals and the inter-individual coefficient of variation is much greater than the intra-individual coefficient of variation, we usually use a 2 (sequential) x 2 (period) single-dose crossover trial design. This reduces the effect of the test period on the test results, thus ensuring the accuracy of the test. It has the advantages of small sample size, high efficiency, high precision, and reduction of the effect of individual differences and the effect of sequence.
Repeat test design
Repeated trial designs include a 3-period repeated crossover design and a 4-period repeated crossover design, which is suitable for both ABE, PBE, and IBE studies. The advantage is that there are more treatment sequences, and the bioequivalence of multiple drugs can be studied at once.
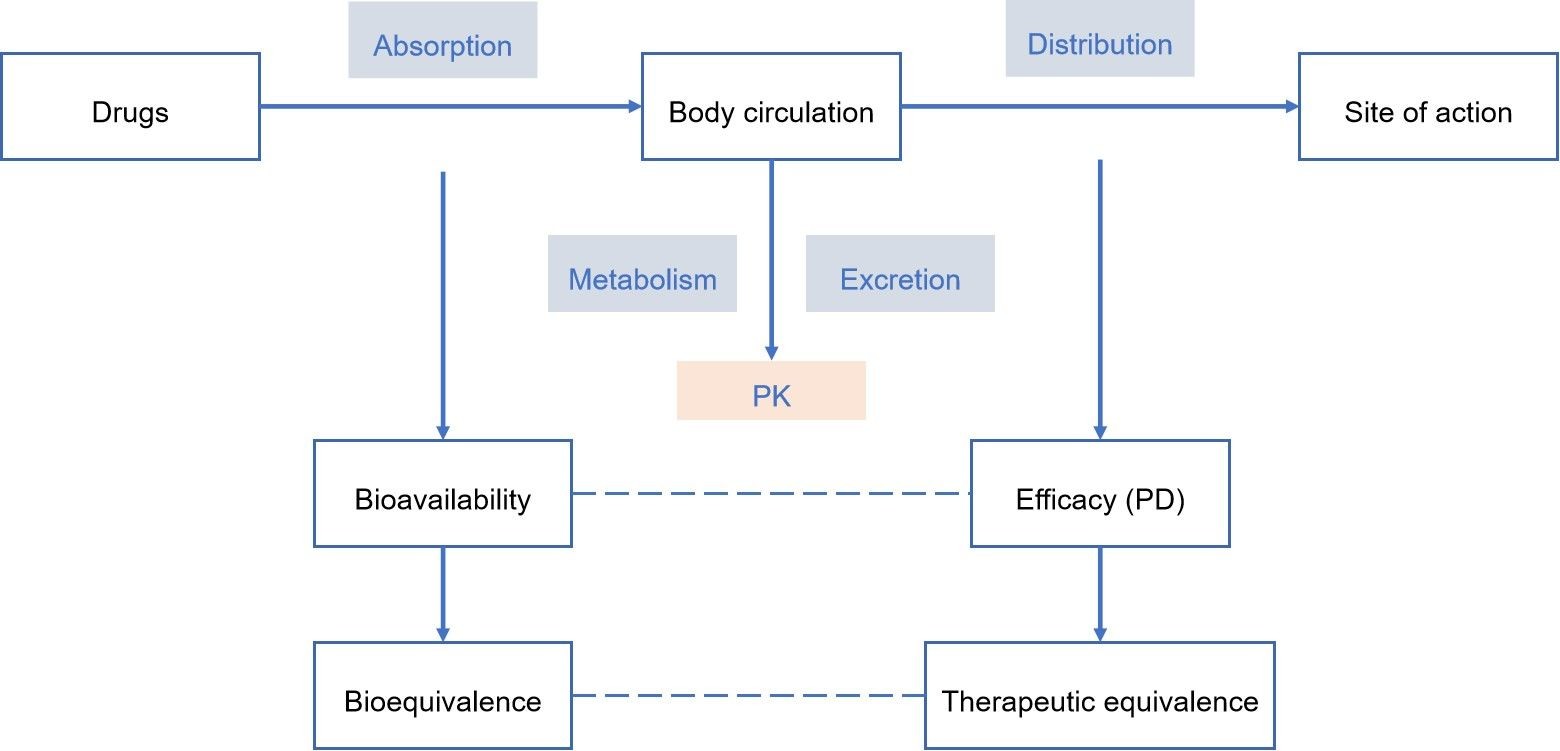
For chemical drug formulations, the active ingredients are monomers, and the quality is easier to control, and the bioequivalence research system is also more complete and mature. However, with the development of new dosage forms and the modernization of herbal medicines, the bioequivalence study of herbal preparations is the focus of quality control of herbal preparations. The same herbal preparation, produced by different manufacturers, with different raw materials from different origins and different production processes, leads to differences in the quality of the herbal preparations produced, so to ensure the efficacy and safety of the herbal preparation products. Lifeasible can carry out bioequivalence analysis on the same preparation produced by different manufacturers. For guidance on the safe, effective, and rational use of herbal medicines, please feel free to contact us for a holistic and comprehensive evaluation of the equivalence and equivalence of herbal medicines through a multi-faceted, multi-level, multi-path, and multi-indicator examination.
For research or industrial raw materials, not for personal medical use!
Related Services


