Induction and Culture of Tobacco Callus
The induction and cultivation of plant callus are of great significance in both basic and applied research in plant science. Through this experiment, we can preliminarily master the aseptic technique of disinfection and inoculation of plant explant materials, the method of callus induction and the method of subculture of callus.
Principle
Plant tissue and cell culture is the process of cultivating isolated plant organs, tissues or cells using aseptic methods. If the plant material used for tissue culture is bacteria-carrying, it is necessary to select a suitable disinfectant to sterilize the surface of the plant explants before inoculation, and obtain sterile material for tissue culture. This is the basic premise and important guarantee for the success of plant tissue culture. Due to the totipotency of plant cells, the explants can be dedifferentiated on a suitable medium to form a cell mass without specific structure and function that can proliferate rapidly - callus. Plant growth regulators such as 2,4-dichlorophenoxyacetic acid (2,4-D) are important factors in inducing explants to form callus.
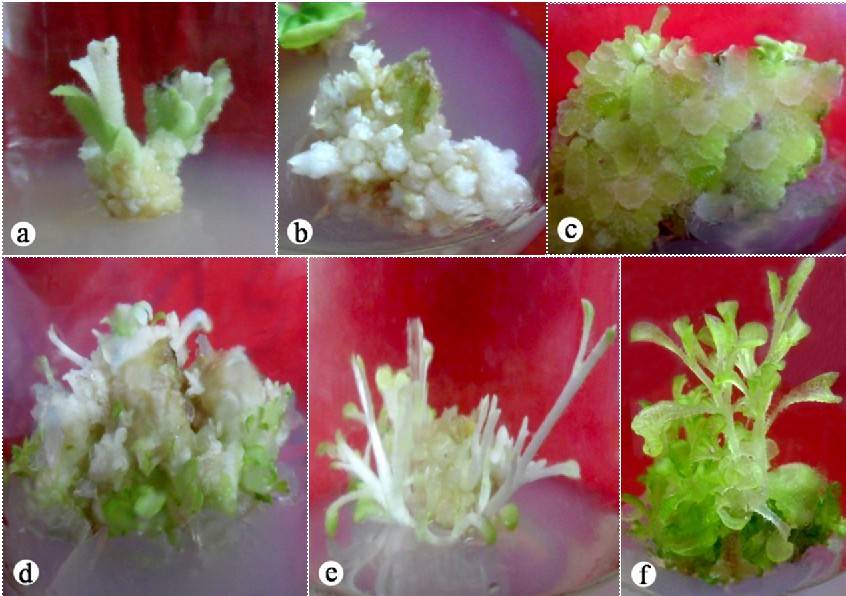 Figure 1. Callus induction and plant regeneration from the calli induced in dark condition (Siddique, A.B., & Islam, S.M. 2018)
Figure 1. Callus induction and plant regeneration from the calli induced in dark condition (Siddique, A.B., & Islam, S.M. 2018)
Procedures
- Before inoculation, wipe the surface of the ultra-clean workbench with 75% ethanol cotton balls. Put the culture medium and inoculation tools on the surface of the ultra-clean workbench, turn on the ultra-clean workbench ultraviolet lamp, irradiate for 20-30 min, and then turn on the air supply. Afterwards, turn off the ultraviolet light, ventilate for 20 minutes, and then turn on the fluorescent light to carry out aseptic operations such as disinfection and inoculation of the explants.
- If potted tobacco seedlings are used, sterilization should be performed first. Take fresh young tobacco leaves and rinse them with tap water. After soaking in 75% ethanol solution for 30 s, transfer to 1‰ mercuric chloride solution with 1-2 drops of Tween-20 and soak for 5 min, 10 min and 15 min respectively. Wash 4 times with sterile water, blot dry with sterile paper, and place in a sterilized petri dish. When using tweezers, scissors and a scalpel, they should be burned on the flame of an alcohol lamp for a while, and after fully cooling, cut the young tobacco leaves into 1.5 cmX1.5 cm square pieces. The above operations are required to be carried out next to the flame of the alcohol lamp. If the sterile seedlings are used, they can be cut directly, eliminating the need for sterilization steps.
- Use sterile tweezers to inoculate tobacco leaves into a petri dish containing callus induction medium MS-1, inoculate 3-4 pieces in each dish, seal it, and label it, indicating relevant information.
- The culture dish inoculated with the explants of the tobacco leaves was placed in an incubator or a tissue culture room for dark culture at a culture temperature of 25°C.
- The formation of tobacco callus on explants, the growth dynamics of callus, the color of callus and the pollution were observed and recorded at intervals (2-5 d).
- Carefully separate the induced callus from the explants, transfer to fresh medium, and after 5-7 subcultures, the tobacco callus with rapid growth and loose texture can be obtained. Induced callus has many uses, such as preparation of protoplasts and cell fusion, mass culture of cells and production of secondary metabolites, genetic transformation of cells, regeneration of plants, preservation of germplasm resources, physiological and biochemical research of cells, etc.
Note
- Mercury chloride (HgCl2) is a highly toxic drug and needs to be prepared and used immediately, so be careful when using it.
- Observe the pollution of tobacco leaf explants after inoculation and culture for 2-6 days, count the number of polluted explants, calculate the pollution rate and determine the reasonable explant sterilization time. If most of the culture material is contaminated, it means that the soaking time of the disinfectant is short. If the inoculation material is not contaminated, but the material has turned yellow or black, and the tissue has become soft, it indicates that the disinfection time may be too long, and the tissue may be destroyed and dead. If the inoculation material is not contaminated and the material grows normally, it indicates that this is the most suitable time for the disinfectant to be disinfected.
Reference
- Siddique, A.B., & Islam, S.M. (2018). Effect of light and dark on callus induction and regeneration in tobacco (Nicotiana tabacum L.). Bangladesh Journal of Botany.
Hot Services & Products
For research or industrial raw materials, not for personal medical use!
 Figure 1. Callus induction and plant regeneration from the calli induced in dark condition (Siddique, A.B., & Islam, S.M. 2018)
Figure 1. Callus induction and plant regeneration from the calli induced in dark condition (Siddique, A.B., & Islam, S.M. 2018)