Purity Assessment of Plant Endoplasmic Reticulum
The isolation and purification of cellular organelles enabled the identification of compartment specific proteins. However, the accuracy of this identification depends on the purity of the target organelle and is also affected by the degree of protein enrichment and contamination. Various methods have been developed and widely used for purity assessment, including microscopic and biochemical methods. To perform these methods, a sufficient level of expertise is required, and only a few dyes specifically for subcellular organelles are commercially available. As an alternative to purity assessment, enzymatic and immunoblot assays have been developed.
Lifeasible provides enzymatic and immune-blot assays for purity assessment with decades of experience. Our experts will answer your questions comprehensively and carefully and help you solve the problems in your studies. We guarantee satisfied and reliable results for our customers all over the world.
Purity Assessment Methods of Plant Endoplasmic Reticulum
- In the experimental design, we use the results of silver staining by SDS-PAGE to judge whether the loading amount of immunoblot samples in the experiment is accurate.
- Lifeasible mainly uses immunoblot and enzyme activity reactions, which are used to detect the relative content of target proteins in the samples.
Purity Assessment Workflows of Plant Endoplasmic Reticulum by Enzymatic Analysis
- Manufactured proteins for enzymatic analysis have been reviewed to indicate contamination from the cytoplasm, plasma membrane, nucleus, mitochondria, and chloroplasts.
- Plasma membrane purity is estimated by comparing H+-ATPase activity with total ATPase activity. The purity of mitochondria has been assessed using a variety of enzymes, including fumarase, aconitase, and cytochrome c oxidase. To assess the purity of chloroplasts, phosphoribokinase activity, and glyceraldehyde 3-phosphate dehydrogenase activity assays have been reported. For ER, NADH: cytochrome c reductase activity is commonly used to assess purity. In addition, the activity of glucose 6-phosphate dehydrogenase can be used to estimate cytosolic contamination.
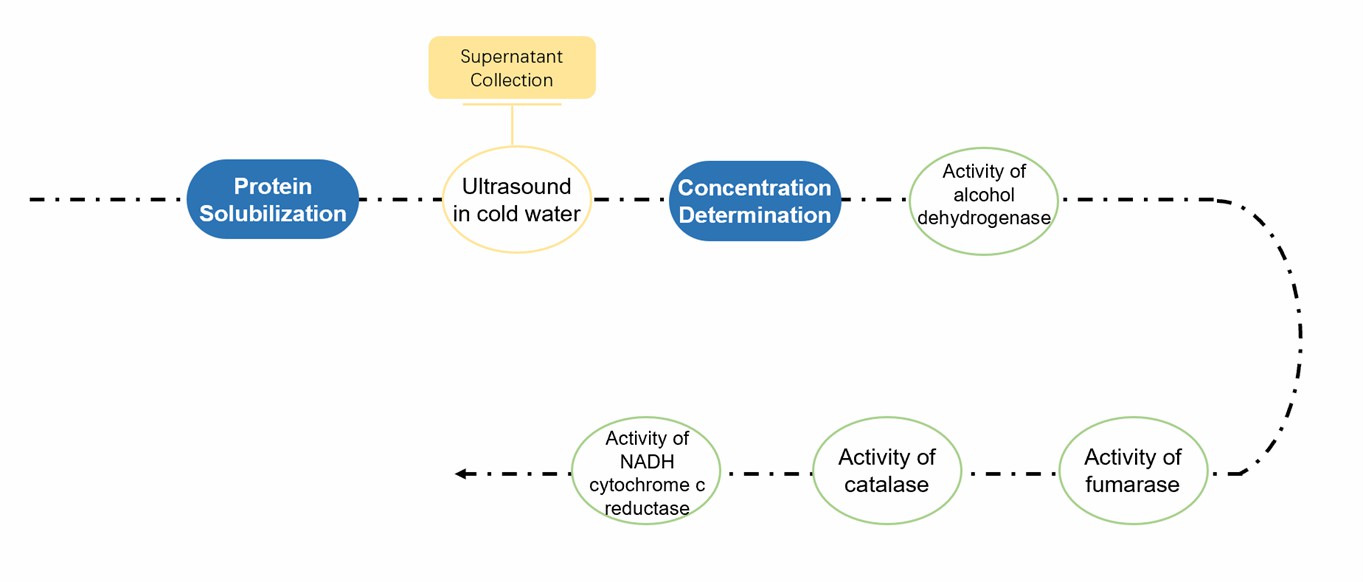 Fig.1 Enzymatic analysis for ER purity assessment.
Fig.1 Enzymatic analysis for ER purity assessment.
Purity Assessment Workflows of Plant Endoplasmic Reticulum by Immunoblot Analysis
- For immunoblot analysis, specific antibodies against subcellular marker proteins are needed.
- We provide Tlg2p, ALP, and Pepl2p, which are used as antibodies to assess contamination from the Golgi, vacuole, and endosomes, respectively. In contrast, ER-resident proteins such as Sec63p, Dpm1p, Kar2p, Sey1p, and Yop1p are used as ER-specific antibodies.
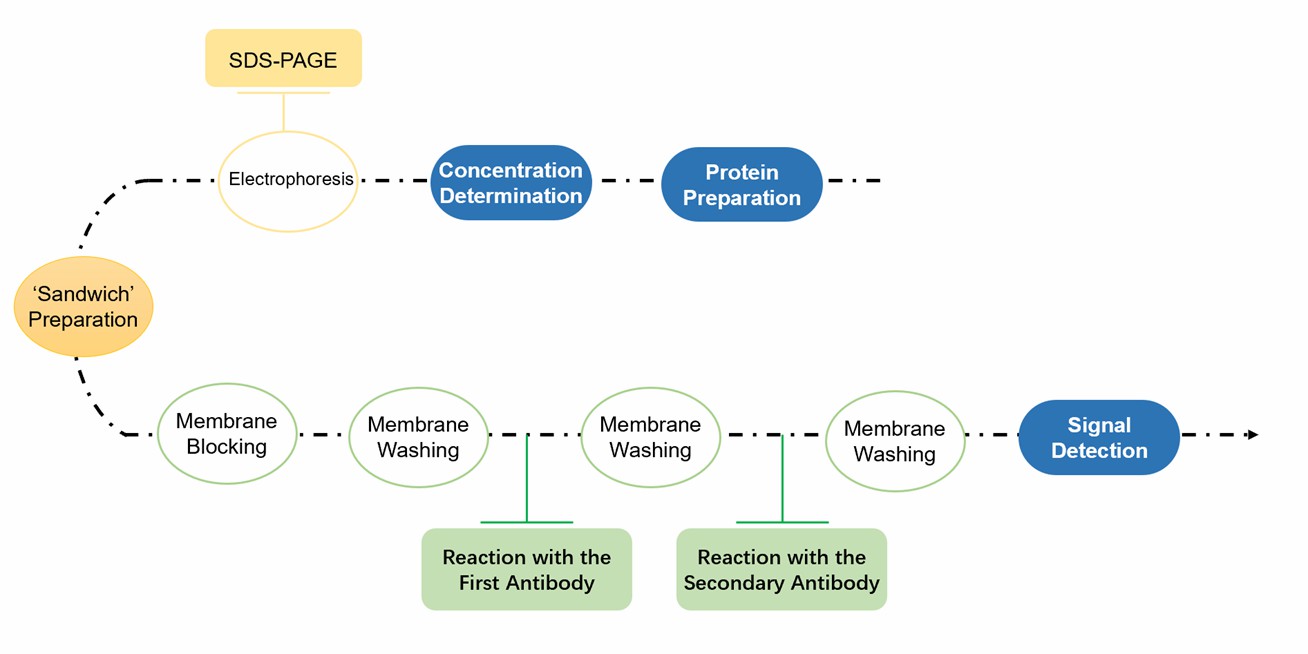 Fig.2 Immunoblot analysis for ER purity assessment.
Fig.2 Immunoblot analysis for ER purity assessment.
Lifeasible offers purity assessment with customized delivery strategies and precise design. Our advanced technical platforms help our clients solve the problems they may encounter in research based on decades of experience. If you are interested in our services or have any questions, please feel free to contact us or make an online inquiry.
For research or industrial raw materials, not for personal medical use!
 Fig.1 Enzymatic analysis for ER purity assessment.
Fig.1 Enzymatic analysis for ER purity assessment. Fig.2 Immunoblot analysis for ER purity assessment.
Fig.2 Immunoblot analysis for ER purity assessment.