Wheat Embryo Culture Technology
Wheat is one of the important food crops, and embryo culture of wheat can not only be used for plant regeneration, but also can break seed dormancy through immature embryo culture to induce dormant seeds to sprout into seedlings and shorten the breeding cycle. Embryo culture can also be used for the rescue of immature embryos. Using the technology of in vitro culture of immature embryos, the interference of asexual nucellar embryos can be eliminated, and hybrid embryos can be obtained, thereby improving the efficiency of hybrid breeding.
Principle
Embryo culture includes isolated embryo culture, ovule culture, ovary culture and endosperm culture. In vitro embryo culture can be divided into mature embryo culture and immature embryo culture. Immature embryo culture refers to the culture of young embryos with embryo structure before the cotyledon stage.
Immature embryos are far from being mature physiologically and morphologically, and their embryonic development requires a more complete artificial synthetic medium, and the stripping technology is very demanding, so it is difficult to culture in vitro. Generally, the older the embryo age, the higher the success rate; on the contrary, the smaller the embryo age, the lower the success rate. Cultivating immature embryos requires more complex medium components, and choosing an appropriate basic medium is one of the first conditions.
The growth of mature embryos does not depend on the nutrients stored in the endosperm. As long as suitable growth conditions are provided and dormancy is broken, they can germinate and form seedlings on a relatively simple medium. Therefore, the medium only needs to contain macroelements such as inorganic salts and sugars. In mature embryo culture, the surface of the fruit or seed (with seed coat) is sterilized with chemicals, the seed embryo is stripped and inoculated on the medium, and can develop into a complete plant under artificial control conditions.
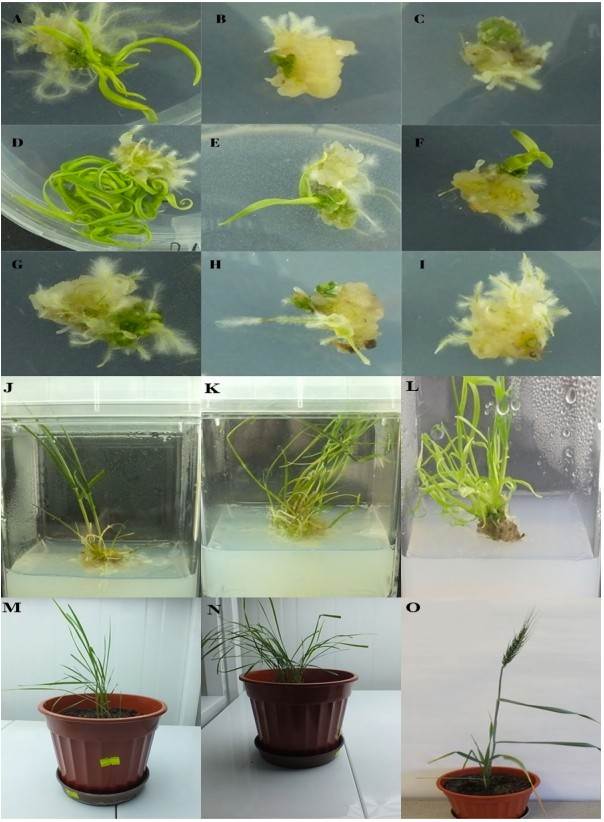 Figure 1. The effect of 6-Benzylaminopurine (BAP) under the different boron stresses on the plant regeneration from callus derived from mature embryos of einkorn and bread wheat. (Ağıl, F.; et al. 2022)
Figure 1. The effect of 6-Benzylaminopurine (BAP) under the different boron stresses on the plant regeneration from callus derived from mature embryos of einkorn and bread wheat. (Ağıl, F.; et al. 2022)
Procedures
A. Wheat Immature Embryo Culture
- Cut off the wheat ears on the wheat plant 12-16 days after the florets in the middle of the ears were pollinated, peel off the developing wheat grains from the chaff one by one, and put them into a sterilized 100 mL beaker. Eliminate milky white grains that are too young and emerald green grains that take too long after pollination, and only keep the grains that have just turned green after pollination (the young embryos are 1-2 mm long).
- Put the tested wheat grains in the beaker on the ultra-clean workbench and sterilize the surface with 70% alcohol for 10 s. Discard the alcohol, and then disinfect with 0.1% mercuric chloride for 5-8 min. Discard the sterilizing solution, and then wash 5 times with sterile distilled water, then it can be used for the isolation and culture of embryos.
- Pick out the immature embryos from the seeds with tweezers under sterile conditions. The immature embryos with scutellum and length less than 2 mm were inoculated on the callus induction medium with the scutellum facing up, and the immature embryos were gently pressed with tweezers to make them closely contact with the medium.
- Put the Erlenmeyer flask inoculated with immature embryos into the incubator, set the temperature at (28+2) °C, and culture under dark conditions or scattered light of 14h/d and light intensity of 20 μmol/(m2·s).
- One week after inoculation of the immature embryos, it can be seen that a small amount of callus tissue has begun to form on the scutellum. After 3 to 4 weeks, the callus was obvious. Under light culture conditions, part of the callus may appear green. At this time, the first subculture can be carried out. When the callus grows to a diameter of more than 1 mm, transfer to the medium of N6 + 2mg/L 2,4-D + 5% sucrose for subculture, and subculture once every 20-30 days.
- When differentiation is required, transfer the callus to the differentiation medium N6 + 0.2 mg/L NAA + 0.5 mg/L KT to produce adventitious bud plants. Cultured under 14 h light conditions every day. The light intensity is 30-40 µmol/(m2·s).
- After 2 to 3 weeks, the green part of the callus differentiated into stems and leaves. However, the regenerated plants at this time are still very weak and cannot be transplanted. They need to be transferred to a medium that does not contain hormones but added 3 mg/L paclobutrazol (PP333), and subcultured 1 or 2 times to strengthen the seedlings.
- Transfer the plants to hormone-free N6 medium to induce rooting.
- When the root system grows to 1-2 cm, open the bottle and harden the seedlings for 3-5 days, then wash the medium attached to the roots of the test-tube seedlings and plant them in the substrate. You can use vermiculite + peat (1:1), you can also use perlite and sand and other cultivation substrates. Avoid direct sunlight for 1-2 weeks after transplanting. Keep the temperature at around 26°C, maintain humidity, and pay attention to ventilation. Move to the field or greenhouse in autumn or early spring.
B. Mature Embryo Culture
- Disinfection and inoculation of explants: First, sterilize the wheat seeds with 70% ethanol for 1 min, then sterilize the wheat seeds with 2% sodium hypochlorite for 15 min, and rinse them with sterile water 4 times. After the surface is sterilized, the seeds are placed in a petri dish, and the embryos are peeled off with a scalpel and tweezers, and placed directly on the culture medium for cultivation.
- Culture medium: Mature embryos have more nutrient accumulation and morphologically differentiated radicle and germ, so the requirements for medium conditions are not high, and it is relatively easy to germinate into seedlings. Due to the difference of explants, the culture of some mature embryos requires a relatively complex medium.
Reference
- Ağıl, F.; et al. In vitro mature embryo culture protocol of einkorn (Triticum monococcum ssp. monococcum) and bread (Triticum aestivum L.) wheat under boron stress. Plant Cell Tiss Organ Cult. 2022, 148: 293–304.
For research or industrial raw materials, not for personal medical use!
 Figure 1. The effect of 6-Benzylaminopurine (BAP) under the different boron stresses on the plant regeneration from callus derived from mature embryos of einkorn and bread wheat. (Ağıl, F.; et al. 2022)
Figure 1. The effect of 6-Benzylaminopurine (BAP) under the different boron stresses on the plant regeneration from callus derived from mature embryos of einkorn and bread wheat. (Ağıl, F.; et al. 2022)