Cannabis sativa L., also called Marijuana, Hemp, and Marihuana, is listed as the five major crop groups with grain, cotton, oil crops, and vegetables. Cannabis sativa L. is widely used and covers areas including textile technology, household board, food processing, medicine and health, etc. The ingredients of cannabis are very complex, mainly including lipids, flavonoids, terpenes, hydrocarbons, non-cyclic macrophenols, alkaloids, silver citrate and cyclic cannabinol, etc. In recent years, the genetic transformation of Cannabis sativa L. has received more and more attention from scholars.

Through the quantitative overexpression of genes related to cannabinoids and other chemical components in Cannabis sativa L., the production of specific compounds can be increased. The gene overexpression services we provide include overexpression of enzymes associated with the oleuric acid metabolic pathway, CBGA and THCA, overexpression service of CBDA synthase-related genes, for specific information, please refer to Gene overexpression service.
RNAi technology refers to the phenomenon of highly conserved, induced by double-stranded RNA (dsRNA), homologous mRNA high-efficiency and specific degradation in the evolutionary process. Through RNAi technology, the silencing of multiple genes in Cannabis sativa L. can be mediated. For specific information and experimental procedures, see RNA Interference (RNAi) service.
VIGS technology is to infect plants by inserting viruses carrying target gene fragments. After the viruses infect Cannabis sativa L., they can induce silencing of Cannabis sativa L. endogenous genes and cause phenotype changes, and then study the function of genes according to phenotype changes. The underlying molecular basis may be post-transcriptional gene silencing. Silencing and functional analysis of target genes in Cannabis sativa L. through VIGS can avoid plant transformation, overcome functional duplication, and work in different genetic backgrounds to achieve thorough analysis of genes. For specific information and experimental procedures, see Virus-Induced Gene Silencing (VIGS) service.
The discovery of the CRISPR/Cas9 system provides us with a very powerful and convenient gene editing tool. By using CRISPR technology, we can achieve knockout of Cannabis sativa L. genes in different ways, including frameshift mutations caused by a single sgRNA, and multiple Deletion of fragments triggered by sgRNA, knockout of non-coding genes, knockout of multiple copies of genes, etc. For specific information and experimental procedures, see CRISPR/Cas9 gene knockout service.
Using the powerful scalability of the CRISPR system, we have developed many methods that can improve gene knock-in efficiency and achieve precise editing of the Cannabis sativa L. genome. Most of CRISPR gene knock-in is done through HDR. However, NHEJ and HDR will occur at the same time due to DNA breaks. Therefore, we have developed different methods to increase the probability of HDR, thereby improving the efficiency of gene knock-in. For details, please refer to CRISPR gene knock-in service.
The CRISPR base editing technology was born in 2016. The current single base editing technology for Cannabis sativa L. can be divided into two types: CBE and ABE, both of which rely on the DNA positioning capabilities of the CRISPR/Cas9 system. During single base editing, the C base deaminase or A base deaminase is located at a specific position in the genome, and it catalyzes the deamination reaction of C or A at a specific position and turns it into U or I. Then it is treated as T or G in the process of DNA replication, Realize the conversion from C to T or A to G. For specific service information and processes, see CRISPR single base editing service.
The dCas system is an important branch of the CRISPR system. There are many ways to participate in the inhibition of gene expression. For the inhibition of Cannabis sativa L. genes, we can provide a variety of gene knockdown solutions, including dCas9 binding to targeted DNA and realize Inhibition of gene transcription through steric hindrance. Gene knockdown can also be achieved by binding a fusion protein to the start site of gene transcription. For details, see CRISPR interference (CRISPRi) service.
CRISPRa technology uses the powerful transformation capabilities of Cas9 and sgRNA to fuse or recruit multiple proteins to enhance gene transcription. For Cannabis sativa L. genes, we can provide VPR technology, SAM technology and Suntag technology to allow the CRISPR system to carry more activation element and achieve a stronger activation effect after synergistic amplification. See CRISPR activation (CRISPRa) service for specific information.
The study of gene function has always been the core subject of biological research. The earliest genetic screening system established through forward genetics is very inefficient and has a huge workload. However, the reverse genetic screening system based on CRISPR technology can completed very low-cost mutation library construction work. The gene mutation library construction technology we provide for Cannabis sativa L. including gene knockout library construction, gene knockdown library construction, gene activation library construction and single-cell sequencing & CRISPR screening system. For specific service details and operating procedures, see CRISPR mutation library construction service.
DNAfree gene editing technology has received extensive attention from the industry in recent years. We can provide DNAfree Cannabis sativa L. genome editing services, including transient expression of CRISPR/Cas9 plasmid DNA, in vitro transcription of CRISPR/Cas9, and pre-assembled ribonucleic acid composed of purified Cas9 protein and sgRNAs Complex, these technologies can avoid the integration of foreign DNA and genome, and can reduce off-target effects. In addition, compared with traditional techniques, these techniques can avoid the use of hybridization or backcrossing to isolate CRISPR/Cas9 chimeras, so they are cheaper and have shorter experimental cycles. For specific service details and operating procedures, see DNAfree gene editing service.
So far, the most advanced and widely used method for the development of genetically modified Cannabis sativa L. is Agrobacterium-mediated. Cannabis sativa L. can be infected by Agrobacterium under natural conditions. Agrobacterium enters the cell through the injured part of the plant and carries itself The T-DNA is introduced into the plant genome. The foreign target gene is transferred and integrated into plant cells through Agrobacterium, and then transformed plants are regenerated through techniques such as tissue culture.
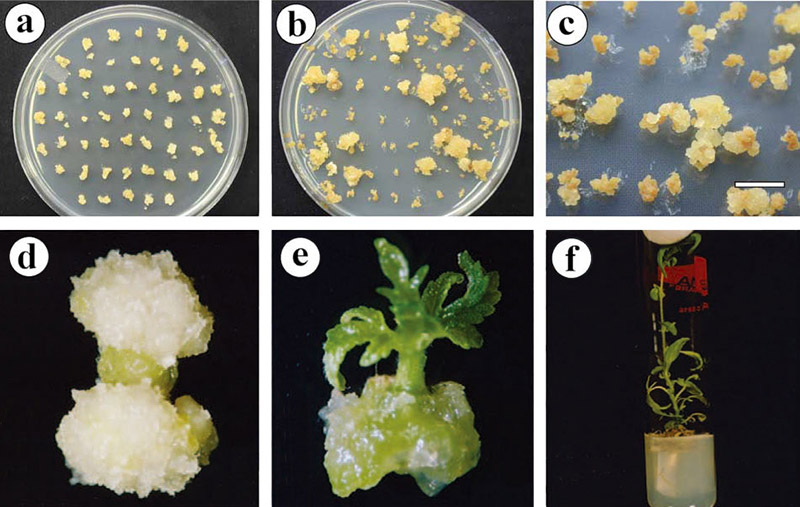 Figure 1. Agrobacterium-mediated transformation of Cannabis sativa L., Medium is composed of MB2.5D, 8 g/L agar, and 0.1 g/L CPR with either 3% sucrose or 2% mannose. (Lisa Burgel, et al. 2020)
Figure 1. Agrobacterium-mediated transformation of Cannabis sativa L., Medium is composed of MB2.5D, 8 g/L agar, and 0.1 g/L CPR with either 3% sucrose or 2% mannose. (Lisa Burgel, et al. 2020)
Lifeasible offers our customers with professional one-stop services, covering all steps including experimental design, vector construction, plasmid transformation, positive transplant screening and testing. Adapting to diverse purposes of all customers, Our gene editing technology can cover multiple Cannabis sativa L.genes (CBDA, CBD, CBGA, CBG, THCA, etc.) And further research on its corresponding phenotype, We can provide the follow-up conversion process with multiple Agrobacterium strains (GV3101, AGL-1, EHA101, EHA105, and C58C1), as well as commercial and customized binary vectors with variant selectable markers (Kanamycin, Hygromycin, Phosphinothricin, G418, etc.) are readily available for your use. Experts at Lifeasible obtain comprehensive knowledge and years of experience to solve technical problems and challenges in Cannabis sativa L. transformation. Our services guarantee the success of your project.
Reference
Lifeasible has established a one-stop service platform for plants. In addition to obtaining customized solutions for plant genetic engineering, customers can also conduct follow-up analysis and research on plants through our analysis platform. The analytical services we provide include but are not limited to the following:
Get Latest Lifeasible News and Updates Directly to Your Inbox