Endoplasmic reticulum (ER) is an important site for synthesizing new peptide chains by a membrane and secreted proteins, and also contains the initial folding modification of new peptide chains. But folding and modification process is complex and error-prone; misfolded proteins in the endoplasmic reticulum quality control (ERQC) system to identify and trap within the endoplasmic reticulum. Once the accumulation of erroneous proteins exceeds the capacity of ER, a series of cellular responses will be triggered to achieve a new ER homeostasis, which is called ER stress response.
Lifeasible, as a leading global company, is committed to helping our customers achieve effective and successful research. We provide analysis of endoplasmic reticulum stress response in plants, including endoplasmic reticulum quality control, unfolded protein response, endoplasmic reticulum quality control, autophagy of endoplasmic reticulum quality control, and others. We always deliver reliable results and reports on time to our customers worldwide.
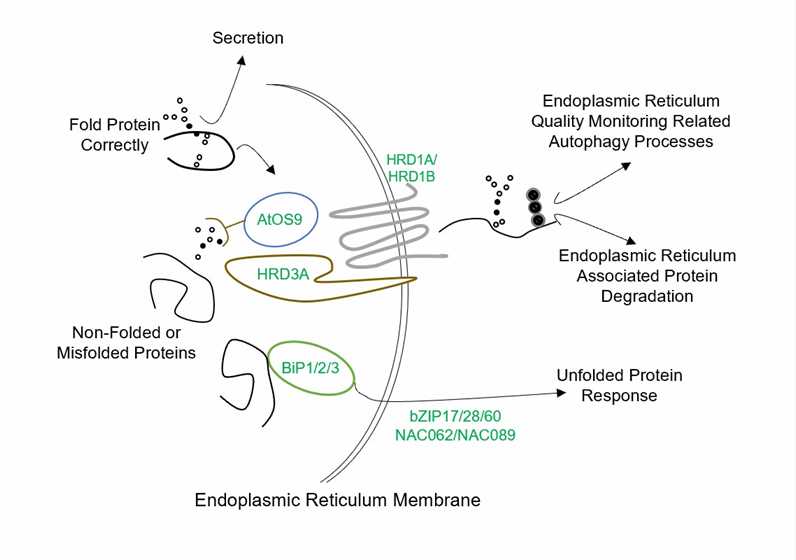 Fig.1 Endoplasmic reticulum quality control system in plants.
Fig.1 Endoplasmic reticulum quality control system in plants.
Lifeasible is always devoted to providing high-quality and satisfactory service to our customers. If you are interested in our services or have any questions, please feel free to contact us or make an online inquiry.
Lifeasible has established a one-stop service platform for plants. In addition to obtaining customized solutions for plant genetic engineering, customers can also conduct follow-up analysis and research on plants through our analysis platform. The analytical services we provide include but are not limited to the following:
Get Latest Lifeasible News and Updates Directly to Your Inbox
Adaptive Evolutionary Mechanism of Plants
February 28, 2025
Unraveling Cotton Development: Insights from Multi-Omics Studies
February 27, 2025