In plant cells, the secretory system delivers many different proteins to four major locations, including the cell wall, vacuole, plasma membrane, and tonoplast. The newly synthesized protein must have a specific conformation in the ER before further downstream delivery by the secretory system. Peptides that fail to fold correctly or never become part of the multi-subunit complex are retained in this compartment. The cells perform 'quality control' on the newly synthesized peptides, allowing the elimination of non-functional proteins.
Lifeasible develops an advanced platform that provides services to customers worldwide covering analysis of protein localization in plant endoplasmic reticulum. We customize featured services according to customer needs with decades of experience in plants. In addition, we deliver satisfactory and reliable results and reports on time to our customers worldwide.
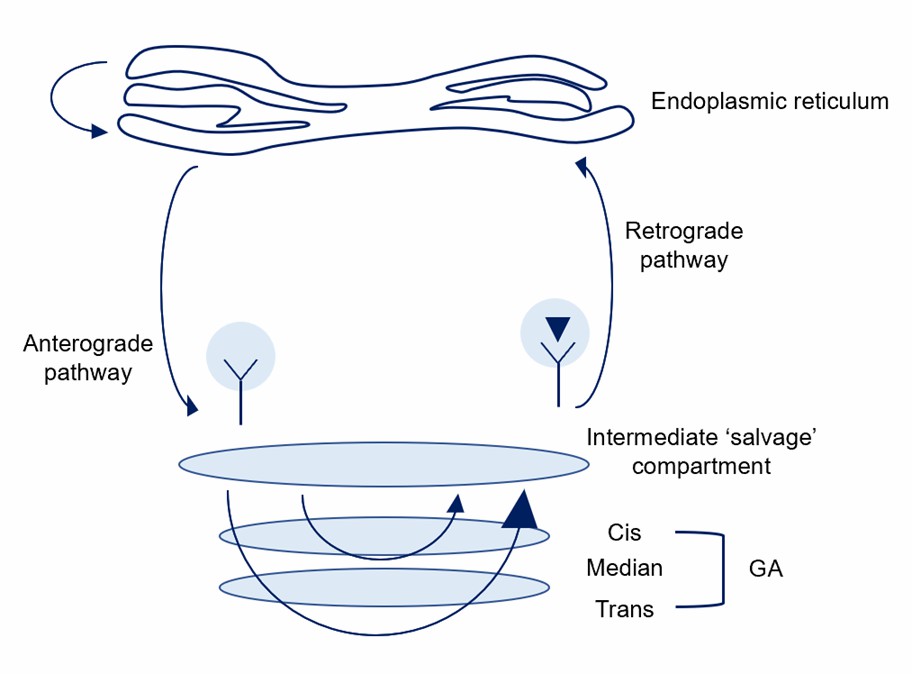 Fig.1 Possible mechanisms of ER protein retention.
Fig.1 Possible mechanisms of ER protein retention.
Lifeasible offers professional services covering analysis of protein localization in the plant ER to meet your research needs. With years of experience in plants, our advanced platforms can help our clients solve various difficulties and conduct research. If you are interested in our services or have any questions, please feel free to contact us or make an online inquiry.
Lifeasible has established a one-stop service platform for plants. In addition to obtaining customized solutions for plant genetic engineering, customers can also conduct follow-up analysis and research on plants through our analysis platform. The analytical services we provide include but are not limited to the following:
Get Latest Lifeasible News and Updates Directly to Your Inbox
Adaptive Evolutionary Mechanism of Plants
February 28, 2025
Unraveling Cotton Development: Insights from Multi-Omics Studies
February 27, 2025