
Honey is a naturally viscous, heterogeneous sweet mixture produced by bees (Apis mellifera) from the nectar of flowers. Honey consists mostly of sugars, which are present in an amorphous and devitrified state. Besides, honey also displays certain thermal behaviors during heat exchange. Due to its appealing taste and high nutritional value, honey has long been a highly consumed natural product. However, over recent years, the phenomenon of adulteration has become a problem for the honey industry. In order to maintain the order of this industry and to safeguard the consumers’ health, it is very important to detect honey adulterants and ensure the quality of honey products.
Globally, authentication of honey covers 5 main aspects (Figure 1). And Lifeasible has a comprehensive portfolio of powerful analytical techniques to help clients determine potential adulterations in honey products.
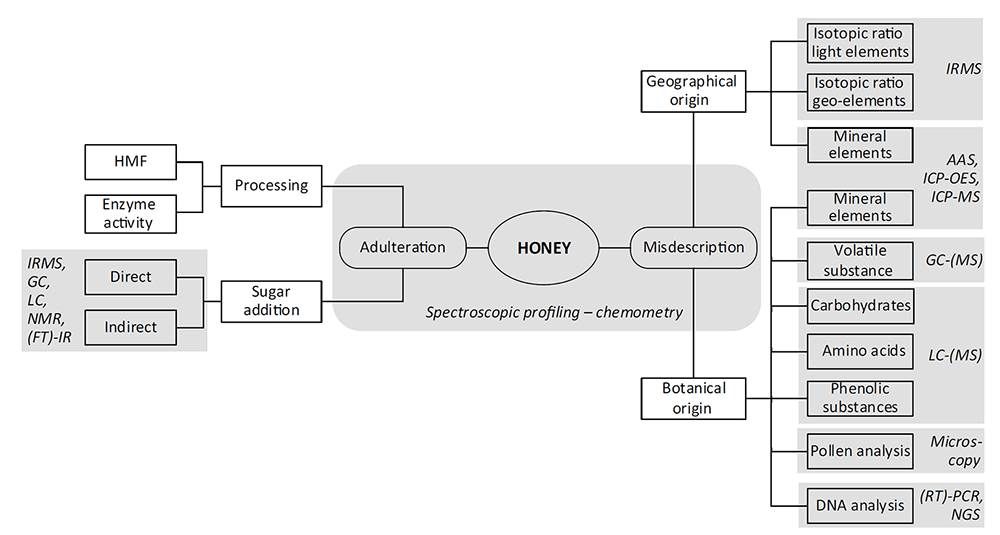 Figure 1. The portfolio of analytical techniques for honey adulteration testing (Ulberth, 2016).
Figure 1. The portfolio of analytical techniques for honey adulteration testing (Ulberth, 2016).
Addition of sugar syrups
Sugars, glucose and fructose, as well as several minor oligosaccharides, are main components of honey. To increase profit, some inexpensive sugars, such as C4 sugars (e.g., corn syrups, sugar cane syrups, and agave syrup) and C3 sugars (e.g., rice, wheat, and beet syrup) are either directly added to honey, or indirectly feed by bees on purpose, which lead to honey adulteration.
Because the majority nectar sources for the honeybees are from C3 plants, therefore the adulteration of C4 sugars can be detected by the technique of stable carbon isotope ratio analysis (SCIRA), which is based on the ratio of 13C to 12C and expressed as 13C/12C = δ13C (‰). The principle of SCIRA is based on the fact that δ13C is different for C3 and C4 plants, where C3 plant species use the Calvin and Benson cycle yielding 13C values near −25‰ (δ13C value ranges from -23‰ to -28‰), while C4 plants mainly use the Hatch-Slack cycle, thus yielding 13C values near −10‰ (δ13C value ranges -9‰ to -15‰). The δ13C of pure honey should be lower than −23.5‰, and the difference between the δ13C values of the honey and its protein should not exceed 1‰.
Besides the SCIRA technique, we also provide chromatographic and spectroscopic approaches for the analysis of both C4 and C3 sugar adulterations. The chromatographic techniques include liquid chromatography coupled to a refractometry detector, high performance anion exchange chromatography coupled to pulse amperometric detection (HPAEC-PAD), gas chromatography coupled to a flame-ionization detector (GC-FID) or mass spectrometry (GC-MS), ultra-high-performance liquid chromatography/quadrupole time-of-flight mass spectrometry (UHPLC/Q-TOF-MS), and so on. Multiple nondestructive and innovative spectroscopic techniques such as Fourier transform infrared (FT-IR) spectroscopy, near infrared (NIR) spectroscopy, nuclear magnetic resonance (NMR) spectroscopy, etc., are available at Lifeasible. Furthermore, we also provide differential scanning calorimetry (DSC), a thermal analysis method, for discrimination of sugar syrups with honey, which is based on the fact that the syrups and honey show significant differences in thermal characteristics, as well as in their amplitudes and positions on the temperature scale.
Misdescription of botanical and/or geographic origin of honey
The mislabeling of both botanical and geographical origins is another type of honey adulteration. Usually, honey is classified as being monofloral when at least 45% of pollen grains arise from a single species and monofloral is predominant in nature. Due to the acknowledged advantages in flavor, taste, and nutritional values, monofloral honey is the most demanded in the market. For this reason, some producers tempt to misdescribe the nectar sources to increase the values of their product. To avoid this type of adulteration, our experts provide melissopalynology and physicochemical analysis to determine botanical and geographical origins of honey. The physicochemical parameters include phenolic profile, volatile compounds, amino acids, proteins, organic acids, minerals and trace elements, DNA markers, and so on, and can be analyzed by chromatography based, spectroscopy based, and DNA based methods.
Undeclared filtration or heat treatment
During the industrial processing of honey, heat treatments, such as heat-assisted filtration or pasteurization are applied, to remove some foreign matters, to sterilize the product, or to delay the onset of crystallization. The application of uncontrolled heat treatments can affect the quality of honey. Our team is capable of precisely determining the diastase activity and hydroxymethylfurfural (HMF) content to assess honey freshness and to avoid the problem of overheating.
Water content
When harvested under high humidity conditions, or artificially added with water, honey products can have fermentation and spoilage problems. Generally, the water content in honey is less than 20%, with the exception of heather honey, for which the limit is 23%. We provide several methods for the determination of honey moisture, including gravimetrical method, refractometry method, Karl Fischer titration (KFT), and so on.
Honey color
Dark honey is usually referred to as “forest honey” and is of higher prices. Thus a very common adulteration of honey products is the addition of a colored dye, dye sulphite ammonia caramel (E150d). The existence of E150d can be determinate by liquid chromatography tandem mass spectrometry (LC-MS/MS).
Lifeasible has a food testing laboratory with state-of-the-art equipment and excellent experts. With our wealth of experience in the food market, we are able to offer you honey adulteration testing services tailored to your needs. If you require further information, please feel free to contact us.
Reference
Lifeasible has established a one-stop service platform for plants. In addition to obtaining customized solutions for plant genetic engineering, customers can also conduct follow-up analysis and research on plants through our analysis platform. The analytical services we provide include but are not limited to the following:
Get Latest Lifeasible News and Updates Directly to Your Inbox
Adaptive Evolutionary Mechanism of Plants
February 28, 2025
Unraveling Cotton Development: Insights from Multi-Omics Studies
February 27, 2025