Proteins are an important source of energy. The average energy density of proteins is about 4 kcal/g or 17 kJ/g, which is comparable to carbohydrates. Proteins contain essential amino acids, such as lysine, tryptophan, methionine, leucine, isoleucine, and valine, which are essential to human health. Moreover, proteins play important roles in regulating human body functions, such as catalyze biological activities, and transport nutrients and other biochemical compounds across cellular membranes. In addition, proteins are major structural components of many natural foods, and can affect the texture, appearance, or stability of food products. Thus, it is important to develop methods for analysis of the identification, concentration, structure, and functional properties of proteins in foods, in order to meet labeling requirements for food quality controls.
Lifeasible has rich experience and cutting-edge facilities in protein analysis of foods. We have developed an advanced platform providing multiple methods for protein analysis in foods, which include, but not limited to:
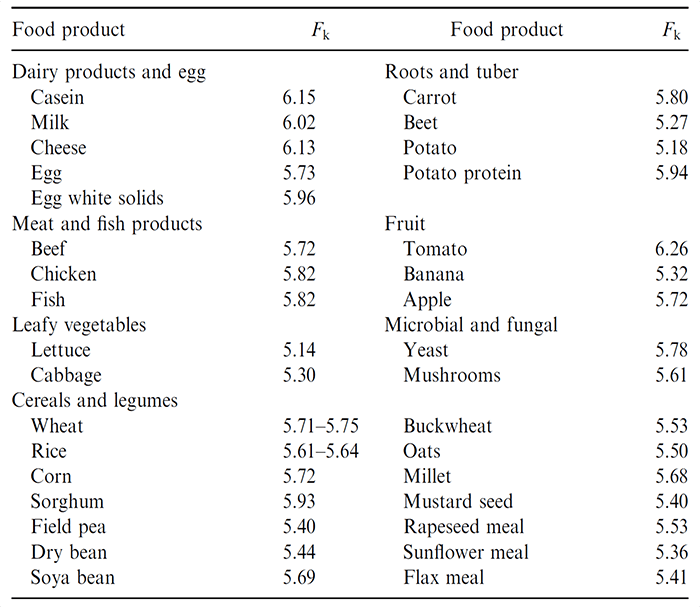
The most commonly used biochemical methods to measure protein content are Kjeldahl method and enhanced Dumas method. The protein concentration is calculated by nitrogen content multiplying a conversion factor (Fk), which varies in different foods (Table 1).
Table 1. Nitrogen-protein conversion (Fk) factors for selected food protein sources (Owusu-Apenten, 2002)
At Lifeasible, a variety of spectrophotometric methods are available to determine protein content in foods, including:
Specific protein components in food and food products can be quantified by chromatography (e.g., HPLC and GC), electrophoresis, immunology, or a combination of these techniques. As the most powerful analytical techniques for the analysis of specific protein components in foods, chromatographic methods are based on the partition coefficients, polarities or sizes of proteins by passing the solution to be analyzed. The separation of proteins in an electric field relies on the basis of their charge density. Immunological methods such as immunoelectrodiffusion and enzyme-linked immunosorbent assay are based on the interaction between an antigen and its corresponding antibody. These are highly specific and sensitive means to identify and quantify minor protein components in complex matrices.
Lifeasible also offers a variety of physical methods for protein content measurement in foods. The physical properties, such as density, refractive index, and light or ultrasonic scattering, are proportional to the concentration of protein in food and food products.
Our state-of-the-art facilities are powerful for determination of protein values. We can provide accurate protein analysis to ensure compliance with all regulatory requirements concerning nutritional declarations, and to avoid extra cost or delays in bringing your products to market. Welcome to contact us for more information.
Reference
Lifeasible has established a one-stop service platform for plants. In addition to obtaining customized solutions for plant genetic engineering, customers can also conduct follow-up analysis and research on plants through our analysis platform. The analytical services we provide include but are not limited to the following:
Get Latest Lifeasible News and Updates Directly to Your Inbox
Mechanisms Regulating Plant Chloroplast Biogenesis
April 15, 2025