Tissue and cell imaging allows the study of structural, functional, and physiological characteristics of plant cells under normal or stress growth conditions. Many technologies have been developed for this purpose. These technologies not only provide anatomical information of plants at tissular, cellular, and subcellular levels, but also allow visualization of spatial-temporal dynamics of fundamental cell processes (e.g., mitosis, morphogenesis, and cytoskeleton dynamics), or the distribution and dynamics of specific molecules (e.g., proteins, nucleic acids, or other metabolites).
Lifeasible, as a leading plant biotechnology company with years of experience in plant cell biology, molecular biology, and physiology, has gained expertise in highly sophisticated up-to-date microscopy technologies. We offer a variety of tissue and cell imaging services, which include, but not limited to:
- Immunohistochemical or Immunocytochemical imaging. This is a highly productive method in which an antigen (protein) within tissues or cells is bound specifically by a fluorescent labeled antibody. The localization of the target protein can be visualized under a light microscope.
- Confocal microscopy. Utilizing a spatial pinhole to block out-of-focus light in image formation, confocal microscopy is an optical imaging technique with increased optical resolution and contrast. It allows examination of biological objects that up to 150 mm in depth, without losing any resolution. Moreover, the confocal laser scanning microscopy (CLSM) allows 3D reconstruction of target molecules in living cells.
- Multiphoton microscopy. Multiphoton microscopy is based on the excitation of fluorescent molecules by two or more photons of red/infrared light emitted by an ultrafast near-infrared laser. It allows examination of thicker specimens, and is less invasive to living samples.
- Atomic force microscopy and scanning probe microscopy. These microscopes are ideal for the imaging of single molecules or macromolecules.
- Raman spectroscopic imaging. This imaging technique allows evaluation of structural and compositional changes in plant tissues and single cells.
- Super-resolution microscopy. Super-resolution microscopy is a term that collectively refers to various techniques that overcome the resolution limitations of the classical far-field optical microscopy systems. Super-resolution microscopy allows visualization of subcellular architectures at the nanoscale. The most popular techniques include structured illumination microscopy, photoactivation localization microscopy, stochastic optical reconstruction, and so on.
- Scanning electron microscopy (SEM). SEM is designed for observation of the microstructure of sample surfaces, evaluation of the sample constituent elements, as well as visualization of the denseness of the tissue surface. Most SEM imaging techniques employ a secondary electron detector under high vacuum, and the samples need to be metal-coated to avoid charging artifacts. Variable pressure-SEM allows examination of uncoated tissues, and provides a flexible range of options for imaging. Focused ion beam–scanning electron microscopy (FIB-SEM) allows reconstruction of the 3D structure of samples at micron-level resolution by sequentially milling the sample surface.
- Transmission electron microscopy (TEM). TEM is a technique in which a beam of electrons is transmitted through a specimen (often an ultrathin section with thickness of less than 100 nm, or a suspension on a grid) to form an image. TEM allows examination of the structures of molecules and materials at the atomic scale.
- Cryo-electron microscopy (Cryo-EM). Cryo-EM is a method for imaging frozen-hydrated specimens at cryogenic temperatures by electron microscopy. Specimens remain in their native state without the need for labeling or fixation, allowing the study of fine structures of cells and protein complexes at atomic resolution.
- Live cell imaging. Live cell imaging is an essential approach for studying the structure, dynamics, and functions of cells in a living plant. A number of advanced techniques for live cell imaging (Figure 1) are listed as follows:
- Microfluidics. Works in combination with plant-on-chip devices, the microfluidics technique allows long-term measurements on growing organs such as root or pollen tubes, under precisely controlled conditions. The system also allows application of treatments during imaging.
- Two-photon excitation microscopy (TPEM). Based on the principle that two low-energy photons being combined in the focal plane can excite the target fluorophore, TPEM enables deep-tissue imaging by evading the light scattering effects of plant cell walls.
- Light sheet fluorescence microscopy (LSFM). LSFM is a mesoscopic method that allows high-speed imaging with very low phototoxicity, as well as the bioimaging of vertically oriented plants. Specifically, samples are illuminated by a thin sheet of light, with controllable thickness in the range of micrometers.
- Total internal reflection fluorescence microscopy (TIRFM)/Variable-angle epifluorescence microscopy (VARM). By adjusting the angle of the laser beam to a super-critical angle (65–67° and 59–61°, respectively), TIRFM/VARM provides improved signal-to-noise ratio (SNR) of samples, and is ideal for the discrimination of membrane- or sub-membrane-resident proteins or metabolites.
- Fluorescence lifetime imaging microscopy (FLIM) and anisotropy. Combined with fluorescence anisotropy measurements, FLIM has recently been established for the in vivo detection of protein-protein interactions, and the compositional characterization of protein complexes.
- Biosensors. Genetically incorporated fluorescence-based sensors for small molecules have enabled the detection and monitoring of metabolites and signaling molecules. The spatiotemporal expression and cellular localization of biosensors can be precisely controlled by the use of specific promoters and targeting sequences, enabling selective analyses of different tissues, cell types, and compartments.
- Phase contrast microscopy. By converting phase shifts to brightness changes in the image, the phase contrast microscopy provides higher image contrasts and reveals many cellular structures that are not visible with simpler bright-field microscopes.
- FlAsH-based live-cell fluorescent imaging. The technology utilizes FlAsH-EDT2, a derivative of the red fluorophore resorufin, to bind the tetracysteine (TC) motif of targeted proteins specifically. The targeted proteins can be visualized in live cells by confocal microscopy.
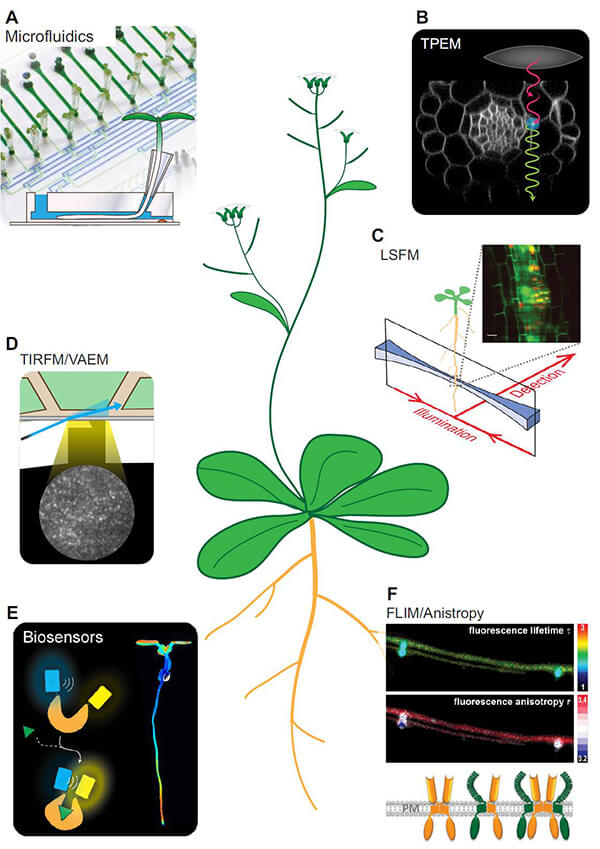 Figure 1. A number of selected state-of-the-art techniques for live cell imaging (Grossmann et al., 2018)
Figure 1. A number of selected state-of-the-art techniques for live cell imaging (Grossmann et al., 2018)
- Mass spectrometry (MS) imaging. MS imaging is the method of scanning a sample of interest and generating images of the intensity distribution of analyte ions. It can be used to investigate the metabolic changes in plant physiological processes, and during the plant-environmental interaction.
- Transparent plant organ method for imaging (TOMEI). TOMEI is a powerful tool for 3D imaging of plant organs at single-cell resolution. It also allows in-depth imaging of plant cell morphology, such as the observation within thick organs. This method requires a clearing reagent containing 2, 20-thiodiethanol.
- Plant-enzyme-assisted (PEA)-CLARITY technique. PEA-CLARITY is a technique that provides improved optical clearing and efficient antibody probe penetration, by using cell wall degrading enzymes and starch hydrolysing enzymes.
Lifeasible is a team with professional knowledge in plant molecular biology, physiology and cell biology. We are devoted to providing our customers worldwide with high quality services at competitive prices. Supported by the most advanced technologies and the intelligence of scientist and experts, our tailored tissue and cell imaging service will ensure the success of your projects.
Reference:
- Grossmann, G., Krebs, M., Maizel, A., Stahl, Y., Jem, V., and Ott, T. (2018). Green light for quantitative live-cell imaging in plants. Journal of Cell Science. 131, 1-14.
For research or industrial raw materials, not for personal medical use!
 Figure 1. A number of selected state-of-the-art techniques for live cell imaging (Grossmann et al., 2018)
Figure 1. A number of selected state-of-the-art techniques for live cell imaging (Grossmann et al., 2018)