Brassica napus L., also known as canola, belongs to the genus Brassica. Brassica napus L. is one of the three major types of rape. It is a compound species derived from Chinese cabbage and cabbage through natural interspecific hybridization and diploid evolution. Brassica napus L. has the highest grain yield among the three types of oilseed rape. Brassica napus L. can not only produce edible oil, but also has rich protein, and it can be used as animal feed. In order to further improve the nutritional value and industrial value of Brassica napus L., reduce the cost of cultivation process, research on genetically modified Brassica napus L. has become a hot field in recent years. Most genetic modification are aim to improve disease resistance, insect resistance, herbicide resistance, salt tolerance, cold tolerance, rapeseed oil production, seed size, and many other traits of Brassica napus L.

Lifeasible offers our customers with professional one-stop services, covering all steps including experimental design, vector construction, plasmid transformation, positive transplant screening and testing. Adapting to diverse purposes of different customers, multiple Agrobacterium strains (C58, LBA4404, EHA105, GV2260, GV3101), as well as commercial and customized binary vectors with variant selectable markers (Kanamycin, Hygromycin, Phosphinothricin, G418, etc.) are available. We offer Brassica napus L. transformation using various genetic engineering technologies as follows:
Gene overexpression is a common technique for studying gene function. The development and utilization of gene overexpression has brought us many conveniences for studying gene function and improving the yield of target products. Through the quantitative overexpression of genes related to oleic acid, linolenic acid, trehalose, and other chemical components in Brassica napus L., the production of specific compounds can be increased. We could help you overexpress many genes including gene BnGPAT6 related to the biosynthetic pathway of substances such as chitin and suberin, ALS gene which is resistant to sulfonylurea (SU) herbicides, the purple acid phosphatase gene BnPAP17, which is related to the resistance of Brassica napus L. to low phosphorus stress, MATH domain gene BnaM154 and genes AT114 and AT148 that affect fatty acid composition of Brassica napus L., and many other genes related to important traits of Brassica napus L.
RNAi technology is widely used in the field of gene editing, it is a phenomenon of specific gene silencing mediated by double-stranded RNA (dsRNA) and involved in specific enzymes. It blocks gene expression at the transcription level, post-transcriptional level, and translation level. Through RNAi technology, we can achieve silencing of multiple genes in Brassica napus L.
Virus induced gene silencing (VIGS) is a genetic technology that inhibits the expression of endogenous genes in plants by inserting recombinant viruses into target gene segments, they can induce plant endogenous gene silencing and cause phenotypic changes, and then study the function of target genes based on phenotypic changes. The VIGS technology is a method of transient transformation and underlying molecular basis may be post-transcriptional gene silencing. Silencing and functional analysis of target genes in Brassica napus L. through VIGS can help our customers save time and achieve valuable information for gene functional analysis. With wealth of experience in VIGS, our scientists in Lifeasible can provide you with customized protocol for VIGS in Brassica napus L.
CRISPR gene knockout technology is currently the most widely used gene knockout technology, it provides us with a very powerful and convenient gene editing tool. As a leading company that has been deeply involved in the field of gene editing for many years, with CRISPR technology, we can knockout Brassica napus L. genes in different ways, including frameshift mutations, multiple deletion of fragments, knockout of non-coding genes, knockout of multiple copies of genes, etc.
CRISPR system has strong scalability, which can be used to develop more useful gene editing tools. we have developed many methods that can improve gene knock-in efficiency and achieve precise editing of the Brassica napus L. genome. For the gene knock-in process, most of CRISPR gene knock-in is done through HDR. However, NHEJ and HDR will occur at the same time due to DNA breaks. Therefore, we have developed different methods to increase the probability of HDR, thereby improving the efficiency of gene knock-in.
CRISPR Single base editing technology is a hot area of life science research today. As a company that has been cultivating gene editing technology for decades, Lifeasible could help you achieve the conversion from C to T or A to G in Brassica napus L. using CBE and ABE, both of which rely on the DNA positioning capabilities of the CRISPR/Cas9 system. During single base editing, the C base deaminase or A base deaminase is located at a specific position in the genome, and it catalyzes the deamination reaction of C or A at a specific position and turns it into U or I. Then it is treated as T or G in the process of DNA replication, realizing the conversion from C to T or A to G.
Sequence-specific control of gene expression on a genome-wide scale is an important approach for understanding gene functions and for engineering genetic regulatory systems. There are many ways to participate in the inhibition of gene expression, one of them is CRISPR Interference (CRISPRi). For the inhibition of Brassica napus L. genes, we can provide a variety of solutions, including dCas9 binding to targeted DNA and realizing Inhibition of gene transcription through steric hindrance. In addition, gene knockdown can also be achieved by recruiting an inhibiting protein to the start site of gene transcription.
CRISPRa technology uses the powerful capabilities of Cas9 and sgRNA to fuse or recruit multiple proteins to enhance gene transcription. For Brassica napus L. genes, we provide VPR technology, SAM technology and Suntag technology to allow the CRISPR system to carry more activation element and achieve a stronger activation effect after synergistic amplification.
The study of gene function has always been the core subject of biological research. The earliest genetic screening system established through forward genetics is very inefficient and has a huge workload. However, the reverse genetic screening system based on CRISPR technology can complete very low-cost mutation library construction work. The gene mutation library construction technology we provide for Brassica napus L. including gene knockout library construction, gene knockdown library construction, and gene activation library construction. Moreover, single-cell sequencing is available for mutation screening.
DNA-free gene editing technology has received extensive attention from the industry in recent years. We provide DNA free Brassica napus L. genome editing services, including transient expression of CRISPR/Cas9 plasmid DNA, in vitro transcription of CRISPR/Cas9, and pre-assembled ribonucleic acid composed of purified Cas9 protein and sgRNAs complex. These technologies can avoid the integration of foreign DNA and genome, and reduce off-target effects. In addition, compared with traditional techniques, these techniques can avoid the use of hybridization or backcrossing to isolate CRISPR/Cas9 chimeras, so they are cheaper and have shorter experimental cycles. Meanwhile, the stable transformation and offspring separation service are available in Lifeasible for obtain of DNA-free gene editing seedlings.
Genetic Transformation Process for Brassica napus L.
Plant transformation refers to the use of recombinant DNA technology, cell tissue culture technology or germplasm system transformation technology to introduce foreign genes into plant cells or tissues and make them fully expressed in recipient cells and then obtain fertile plants for stable expression of foreign genes. So far, the most advanced and widely used method for the development of genetically modified Brassica napus L. is Agrobacterium-mediated hypocotyl transformation.
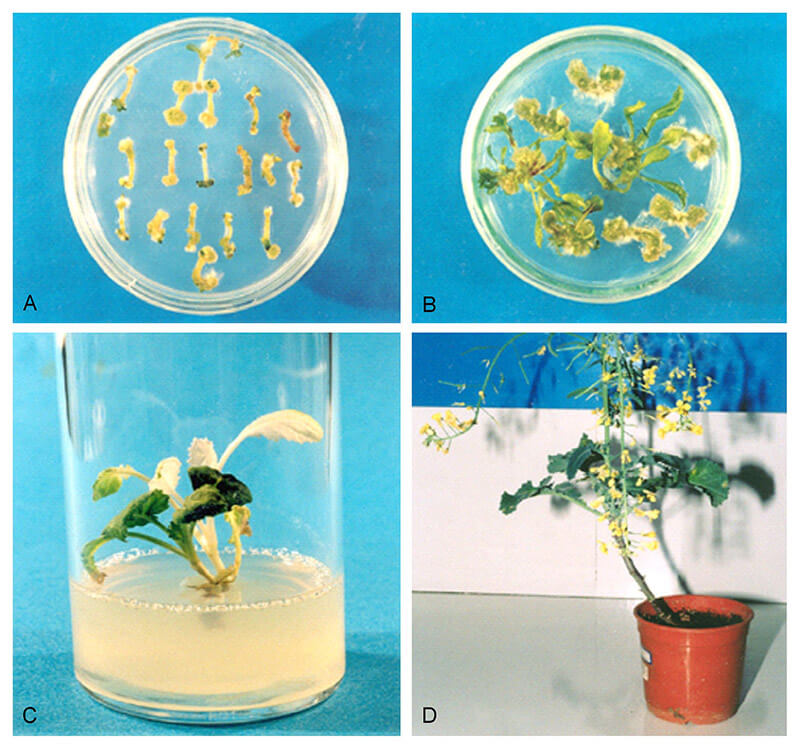 Figure 1. Agrobacterium mediated transformation of Brassica napus L. by infecting hypocotyl by immersing for 10 min in the bacterial inoculum, (A) Shoot regeneration from 7-d-old explants on shoot induction medium (SIM), (B) High frequency of shoot regeneration from 21-d-old explants on SIM, (C) Transformed (green) and untransformed (white) shoot regenerated from hypocotyl on the selection medium supplemented with kanamycin, (D) Establishment of transformed plantlets in the soil and appearance of normal flowers and seeds. (Jonoubi P, et al. 2005)
Figure 1. Agrobacterium mediated transformation of Brassica napus L. by infecting hypocotyl by immersing for 10 min in the bacterial inoculum, (A) Shoot regeneration from 7-d-old explants on shoot induction medium (SIM), (B) High frequency of shoot regeneration from 21-d-old explants on SIM, (C) Transformed (green) and untransformed (white) shoot regenerated from hypocotyl on the selection medium supplemented with kanamycin, (D) Establishment of transformed plantlets in the soil and appearance of normal flowers and seeds. (Jonoubi P, et al. 2005)
Experts at Lifeasible obtain comprehensive knowledge and years of experience to solve technical problems and challenges in Brassica napus L. transformation. our services guarantee the success of your project. For more information or any inquiry requirements, please contact Lifeasible.
Reference