The milk fat peroxide value indirectly reflects the freshness of the milk as well as the quality of the milk. Lifeasible offers services to help determine the milk fat peroxide value.
Milk and milk products contain about 23-25% unsaturated fatty acids, which are sensitive biochemical compounds towards auto-oxidation. The fatty acid profile of milk and milk products can also be altered by lipid oxidation. As the milk and milk products are stored longer, the more peroxidized fat in the milk and more free fatty acids. The peroxide level of milk fat is generally measured to determine the freshness of the milk. Commonly used methods include peroxide value determination, free fatty acid determination, and conjugated dienes determination. Here, we offer services to help determine the freshness of milk and milk products by measuring the peroxide value of milk fat.
 Fig. 1 Check the freshness of the milk.
Fig. 1 Check the freshness of the milk.
Lifeasible offers two methods to help detect peroxide in milk fat, the iron (II) chloride/ammonium thiocyanate method and the iodine/thiosulfate method.
Iron (II) chloride/ammonium thiocyanate method
This method is mainly for anhydrous fat samples with peroxide values between 0.5 mmol to 1.3 mmol of oxygen per kilogram.
The method is based on photometric determination. This method requires extracting milk and milk products to obtain anhydrous fat. In the case of butter and other high-fat products, the fat fraction is physically separated. Then, the anhydrous fat is dissolved in a mixture of methanol/1-decanol/hexane. After the addition of iron (II) chloride and ammonium thiocyanate, the iron (II) is oxidized, forming a red iron (III) complex with ammonium thiocyanate. The red iron (III) complex is measured at 500 nm absorbance to determine the peroxide value.
Operation flow:
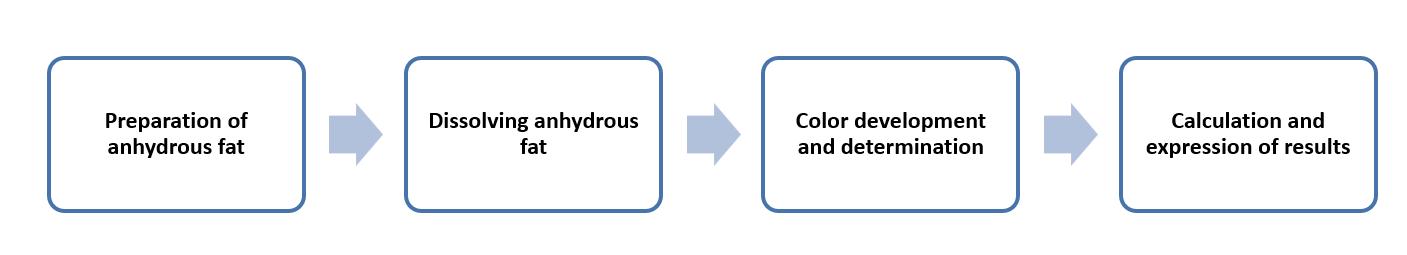
Main reference standard:
ISO 3976:2006
This method is mainly for anhydrous fat samples with peroxide values of more than 1.3 mmol of oxygen per kilogram.
The method is based on the titration method and starch is used as an indicator. The method still requires the fat to be dissolved first. Then, add the standard KI solution. The peroxide oxidizes I- to I2. The amount of I2 generated is subsequently titrated using standard sodium thiosulfate (Na2S2O3). The peroxide value is calculated based on the amount of Na2S2O3 used.
Operation flow:
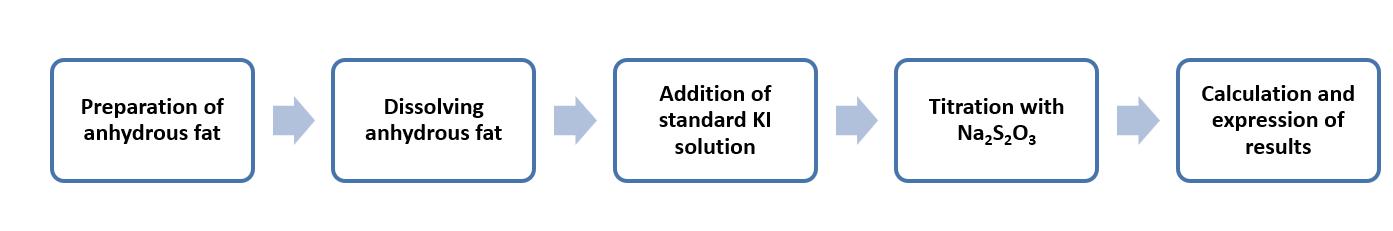
Main reference standard:
AOAC official method 965.33-1969: Peroxide value of oils and fats
Light can be used as an energy source for oxidation, accelerating the rate of oxidation. The shorter the wavelength of light, the stronger the ability to promote oxidation. Please ensure that the sample is not oxidized during delivery.
Lifeasible offers professional milk fat peroxide value determination services to identify the freshness of milk and milk products. Our detection methods refer to international standard methods to ensure professionalism and practicality. We also provide other services to determine the level of milk peroxide, such as the measurement of free fatty acid content in milk. Please contact us for milk-related testing; we will be your trustworthy partner.
References
Lifeasible has established a one-stop service platform for plants. In addition to obtaining customized solutions for plant genetic engineering, customers can also conduct follow-up analysis and research on plants through our analysis platform. The analytical services we provide include but are not limited to the following:
Get Latest Lifeasible News and Updates Directly to Your Inbox
Adaptive Evolutionary Mechanism of Plants
February 28, 2025
Unraveling Cotton Development: Insights from Multi-Omics Studies
February 27, 2025