The endoplasmic reticulum-associated degradation (ERAD) machinery clears the misfolded protein substrate through the cytoplasmic 26S proteasome. In plants, ERAD not only plays an important role in normal growth and development but also is related to the process of tolerance to stress. The process consists of four steps, recognition of misfolded proteins, inversion of proteins located in the ER lumen and ER membrane to the cytoplasm, ubiquitination modification under the action of specific E1, E2, and E3 ligases, and degradation by 26S proteasome.
Lifeasible makes full use of our specialized libraries, selection strategies, and maturation techniques to analyze the four steps in the process of endoplasmic reticulum-associated degradation. With advanced technology and experienced staff, we provide comprehensive ER stress response services for global clients to support their research.
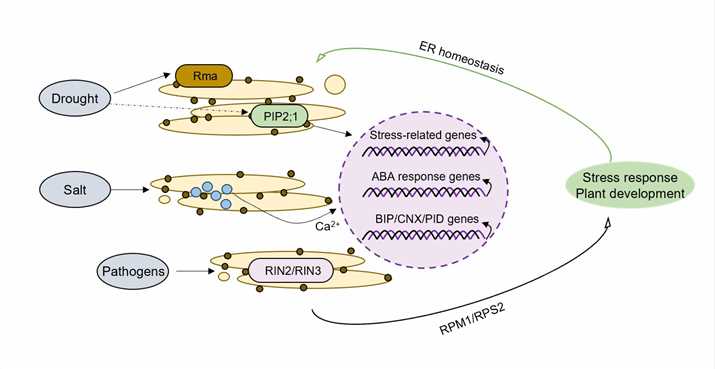 Fig.1. Endoplasmic reticulum-associated degradation plays crucial roles in biotic and abiotic stress responses.
Fig.1. Endoplasmic reticulum-associated degradation plays crucial roles in biotic and abiotic stress responses.
Lifeasible offers professional services covering endoplasmic reticulum-associated degradation to meet your research needs. With years of experience in plant sciences, our advanced platforms can help our clients solve various difficulties and conduct research. If you are interested in our services or have any questions, please feel free to contact us or make an online inquiry.
Lifeasible has established a one-stop service platform for plants. In addition to obtaining customized solutions for plant genetic engineering, customers can also conduct follow-up analysis and research on plants through our analysis platform. The analytical services we provide include but are not limited to the following:
Get Latest Lifeasible News and Updates Directly to Your Inbox
Adaptive Evolutionary Mechanism of Plants
February 28, 2025
Unraveling Cotton Development: Insights from Multi-Omics Studies
February 27, 2025