Mechanism Analysis of Posttranslational Modification Mediated by Effector Proteins
Plant nematode effector proteins may also be involved in post-translational modifications by interacting with proteins related to the plant post-translational modification pathway (PTMs). These modifications include phosphorylation, ubiquitination, glycosylation, proteolysis, and histone modification. It was found that effector proteins in plant nematodes regulate host immune response through auto-glycation, proteolysis, and ubiquitination.
Lifeasible provides mechanism analysis of posttranslational modification mediated by effector proteins to help our customers worldwide in plant science research. Our experts will answer your questions comprehensively and carefully and help you solve the problems in your studies. We guarantee satisfied and reliable results for our customers all over the world.
Mechanism Analysis of Auto-Glycation Mediated by Effector Proteins
- Glycosylation and proteolysis are the two important modes of protein modification in plant cells. Pathogens can secrete glycoproteins directly into host plants or utilize the host post-translational machinery to form glycosylated effectors, avoiding plant immunity and promoting the pathogenesis of pathogens.
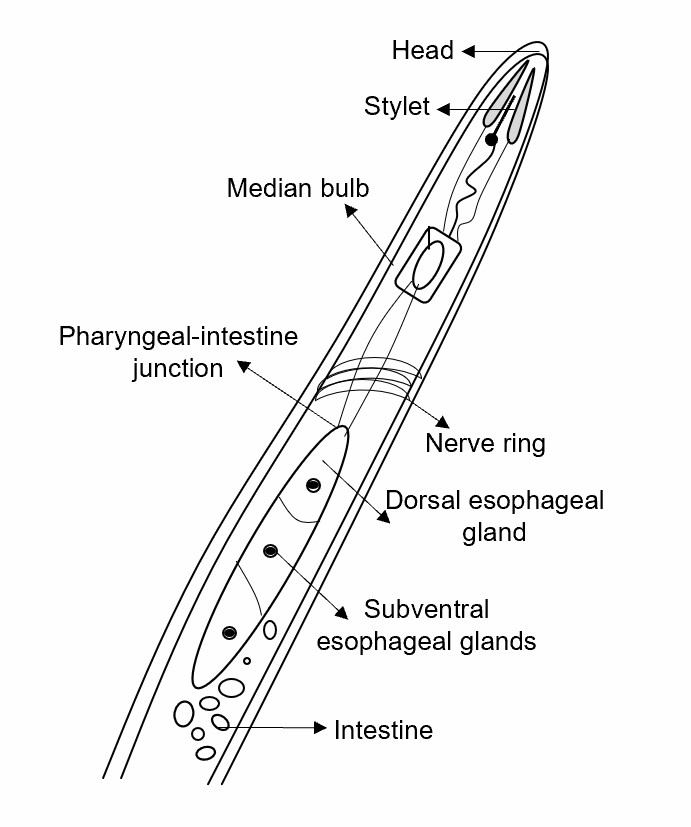 Fig.1 Expression patterns of MgGPP in plant nematodes.
Fig.1 Expression patterns of MgGPP in plant nematodes.
- Lifeasible provides an analysis of auto-glycation and proteolysis secreted by plant nematodes, including MgGPP in root-knot nematodes, which utilizes the host's post-transcriptional modification mechanism to carry out its glycosylation and proteolysis. MgGPP, after N-terminal glycosylation, can inhibit programmed cell death.
- We also provide the biotechnology that enables this process, such as gene amplification and sequence analysis, plasmid construction and generation of transgenic plants, southern blot analysis, developmental expression analysis and in situ hybridization, anti-MgGPP polyclonal serum production and immunofluorescence localization, subcellular localization, western blot analysis, N-glycosylation analysis, proteolysis analysis, infection assay, and cell death assay.
Mechanism Analysis of Ubiquitination Mediated by Effector Proteins
- The ubiquitin-proteasome system plays a significant role in many plants' physiological processes by the removal of intracellular proteins. The ubiquitin pathway involves the sequential action of E1 (ubiquitin-activating enzyme), E2 (ubiquitin-conjugating enzyme), and E3 (ubiquitin ligase) enzymes to covalently link ubiquitin to E3-specified substrate proteins which are then transported to the proteasome for degradation.
- A growing body of evidence has suggested that pathogens may use effectors to manipulate the host ubiquitin pathway to promote pathogenesis, during which effectors may either hijack the host ubiquitin system or themselves possess E3 activity to ubiquitinate host defense-related factors for degradation.
- We provide an analysis of ubiquitination secreted by plant nematodes, including GrUBCEP12 and RHA1B, which is an effector protein with E3 ubiquitin ligase activity, inhibits the hypersensitivity response and inhibits the PAMP-triggered immunity response stimulated by flg22 in an E3 ligase independent manner.
Lifeasible offers mechanism analysis of effector proteins in plant nematodes with customized delivery strategies and precise design. Our advanced technical platforms help our clients solve the problems they may encounter in research based on decades of experience. If you are interested in our services or have any questions, please feel free to contact us or make an online inquiry.
For research or industrial raw materials, not for personal medical use!
 Fig.1 Expression patterns of MgGPP in plant nematodes.
Fig.1 Expression patterns of MgGPP in plant nematodes.