Nematode Staining in Diseased Root Tissue
Staining nematodes in root tissue is a routine method used in most nematology laboratories worldwide. The ability to visualize parasitic nematodes in root tissue is critical for many areas of research in plant nematology, including assessing host plant resistance, elucidating the development and life cycle of nematodes, and evaluating the effectiveness of biological control products and traditional nematicides. Staining with acid fuchsin and lactic acid phenol are the most widely used techniques, and more rapid methods have also been developed.
Powered by our professional scientists and their years of field experience, Lifeasible can provide nematode staining in diseased root tissue. With our cutting-edge platforms, we can achieve staining by sodium hypochlorite acid fuschin method, red food coloring method, and so on.
Sodium Hypochlorite Acid Fuschin Method
- It is found that potassium hydroxide (KOH), nitric acid, hydrogen peroxide (H2O2), or sodium hypochlorite (NaOCl) are effective at removing roots, depending on the type of tissue to be removed. Alkaline H: O is the best bleach for pigment roots. However, overuse of NaOCI bleach often results in poor tissue staining.
- Lifeasible provides nematode staining in diseased root tissue with the sodium hypochlorite acid fuschin method, including clearing root tissues, staining nematodes in root, and distaining the roots.
| Steps |
Operation Methods |
| Clearing Root Tissues |
- Wash roots with water and place them in a 150 ml beaker. Large root systems may be cut into sections for staining.
- Add 50 ml of tap water and an appropriate amount of chlorine bleach (5.25% NaOCl) to clear the root tissue. Soak the roots for 4 minutes in the NaOCl solution and agitate occasionally.
|
| Staining Nematodes in Root |
- Rinse the roots with running tap water for about 45 seconds and then immerse them in water for 15 minutes to remove any residual NaOCl, which may affect staining with acid fuschin.
- Drain the water and transfer the roots to a glass beaker with 30-50 ml of tap water.
- Add 1 ml of stock acid-fuschin stain solution to the water and boil for about 30 seconds on a hotplate or in a microwave oven. To prepare a stock acid-fuschin solution, dissolve 3.5 g acid fuschin in 250 ml acetic acid and 750 ml distilled water.
- Cool the solution to room temperature, drain the stain solution, and rinse the roots in running tap water.
|
| Distaining the Roots |
- Destain the roots by boiling in 20-30 ml of glycerin acidified with a few drops of 5 N HCl.
- Distribute the roots in a small amount of glycerin on a dish cover, gently press against the cover with a dish bottom and observe under a dissecting microscope. A pair of glass plates may be used instead of Petri dishes.
|
Red Food Coloring Method
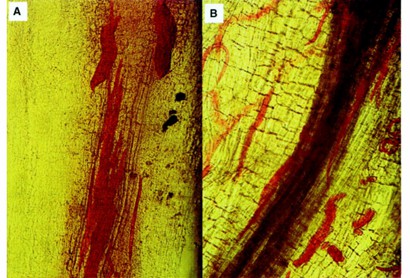 Fig.1 Nematodes ang eggs stained with red food color in diseased root tissue. (Thies JA et al., 2002)
Fig.1 Nematodes ang eggs stained with red food color in diseased root tissue. (Thies JA et al., 2002)
- Staining root-knot nematode egg masses present on root surfaces with phloxine B is another technique that is widely used in plant nematology. Protective eyewear, chemical-resistant gloves, and a NIOSH / MSHA-approved respirator are recommended as personal safety protective equipment when using phloxine B.
- We provide nematode egg masses staining in diseased root tissue with the red food coloring method. And the ingredients of red food color are H2O, propylene glycol, FD&C Reds 40 and 3, propylparaben, and others.
Lifeasible offers nematode staining in diseased root tissue for your research convenience. Our goal is to help our clients achieve meaningful results through powerful and consistent approaches. If you are interested in our services or have any questions, please feel free to contact us or make an online inquiry.
Reference
- Thies JA, et al. (2002). "Red food coloring stain: new, safer procedures for staining nematodes in roots and egg masses on root surfaces." J Nematol. 34 (2), 179-81.
For research or industrial raw materials, not for personal medical use!
 Fig.1 Nematodes ang eggs stained with red food color in diseased root tissue. (Thies JA et al., 2002)
Fig.1 Nematodes ang eggs stained with red food color in diseased root tissue. (Thies JA et al., 2002)