Lifeasible provides customers with customized protoplast regeneration and transformation solutions, ensuring efficient and high-success rate services. Our comprehensive plant service platform provides a one-stop experience and enables seamless connection between upstream and downstream research for customers.
Plants have significant reprogramming potential that helps plants regenerate from organs, tissues, and even individual cells. Protoplasts exhibit significant de-differentiation capacity, and cultured protoplasts can form cell walls and undergo cell division, thus permitting regeneration of the entire plant.
Given the advantages of improved synchronization of single-cell initiation without sexual reproduction, protoplast regeneration techniques have been widely used for genetic engineering and genome editing in plants. For example, clusters of regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated protein 9 (Cas9) systems have been transiently expressed in plant protoplasts, and genome-edited protoplasts have been regenerated into individual plants. In addition, DNA-free genome editing has been developed by delivering pre-assembled Cas9-gRNA ribonucleoprotein (RNP) into protoplasts derived from somatic cell tissues.
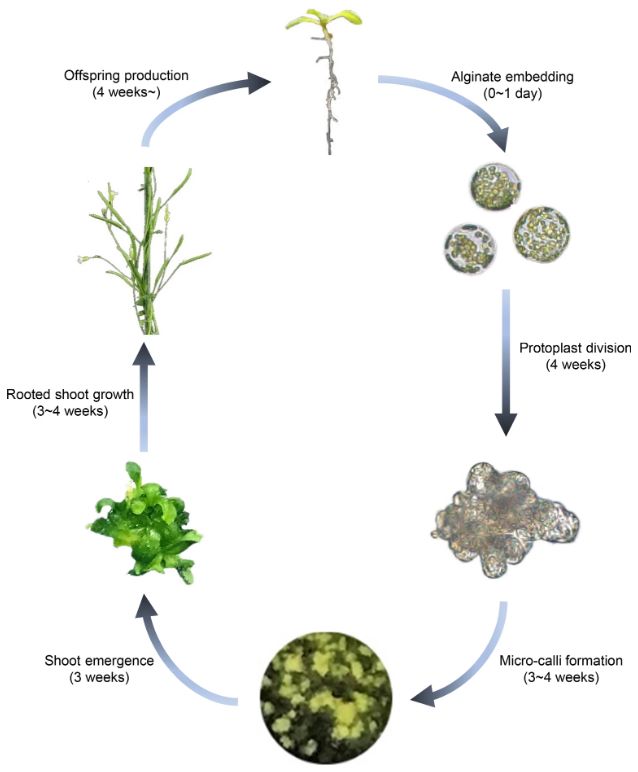 Figure 1. Schematic overview of the Arabidopsis protoplast regeneration process. (Jeong, Y.Y., et al., 2021)
Figure 1. Schematic overview of the Arabidopsis protoplast regeneration process. (Jeong, Y.Y., et al., 2021)
Unlike tissue explant-derived plant regeneration, the molecular processes involved in cell fate transitions during protoplast regeneration are largely unknown. Lifeasible draws on its many years of expertise to provide essential research services on the critical processes involved in protoplast regeneration, including cell wall restoration, cell cycle re-entry, healing tissue formation, pluripotency acquisition, and regeneration from scratch. We aim to understand the molecular mechanisms of protoplast regeneration to advance further plant cell-based biotechnology applications, such as genome editing and somatic cell hybridization.
We provide efficient protoplast regeneration and transformation programs for various plants, including cucumber, sweet potato, sugar beet, soybean, rose, Arabidopsis, tobacco, lettuce, rice, petunia, etc.
Within 2-4 days of culture, protoplasts lose their characteristic spherical shape, a sign of new cell wall regeneration. We offer Calcofluwhite ST staining and various electron microscopy techniques to analyze protoplast cell wall regeneration more reliably and directly.
We have strict control over the conditions of cell division and healing tissue formation, such as the type of plant, the material used to isolate the protoplasts, the quality of the protoplasts, the composition of the culture medium, and the culture conditions. We ensure successful cell division, growth, and healing tissue formation.
Healing tissues formed from protoplasts can be transferred directly to a differentiation medium for one-step seedling formation. We use solid MS medium supplemented with growth hormones and cytokinins to increase regeneration efficiency. We have successfully developed protoplast regeneration methods in various plant species, including liquid culture, alginate embedding methods, and others.
We offer electroporation or polyethylene glycol (PEG)-mediated transient transformation of protoplasts. Enzymatic removal of the plant cell wall allows the introduction of exogenous DNA, RNA, or proteins into protoplasts by PEG treatment or electroporation. The more common PEG-mediated transformation requires less material than electroporation and can achieve high transformation rates, e.g., up to 90% for petunia leaf protoplasts.
01
High protoplast transformation efficiency
02
Highly reproducible and efficient
03
Personalized protoplast regeneration protocol
04
One-stop service platform
Lifeasible recognizes that each project has unique challenges and goals, providing tailored solutions for protoplast regeneration and transformation. Whether it's basic research or crop improvement, our team of experts can develop customized strategies to ensure the success and efficiency of each endeavor.
Contact us today to learn more about our services.
Lifeasible provides a variety of services to support customers' botanical research, including plant analysis services, genetic engineering services, genetic transformation services, etc.
Reference